Journal of Nanobiotechnology volume 23, Article number: 429 (2025) Cite this article
The nucleoside analogue 6-thio-2'-deoxyguanosine (6-thio-dG, also known as THIO) is a telomere-targeting agent with important clinical potency. It can selectively kill telomerase-positive tumor cells. We previously reported that THIO could successfully induce immunogenic cell death (ICD) in multiple mouse tumor cell lines. In this study, we further explored the potential impact of THIO on remodeling the tumor microenvironment, regulating anti-tumor immune responses, and its possible synergistic effects with other therapeutic methods, such as tumor vaccines. Our results showed that THIO could also induce ICD in various human tumor cell lines. The induction of ICD in tumor cells promoted the migration and maturation of antigen-presenting cells. Administration of THIO significantly inhibited the growth of established CT26 and TC-1 tumors in mice. Meanwhile, it enhanced the anti-tumor CTL response and reduced the levels of immunosuppressive myeloid-derived suppressor cells (MDSCs) in both the spleen and tumor tissues. Additionally, THIO had a direct inhibitory effect on the proliferation and differentiation of MDSCs. Moreover, when combined with bacterial biomimetic vesicles or a nanovaccine, such as THIO with BBV or different Q11-tumor antigen peptide nanofibers, it exhibited enhanced anti-tumor effects and immune responses compared to monotherapy in either “immune hot” TC-1 tumors or “immune cold” B16-F10 tumors. In summary, THIO has the ability to remodel the tumor microenvironment, exert a specific killing effect on tumor cells, and effectively cooperate with tumor vaccines. This broadens the anti-tumor mechanisms of THIO and provides a promising strategy for improving anti-tumor immunotherapies.

Malignant tumors have always been serious threats to human life and health [1]. In recent years, therapeutic strategies targeting telomerase have garnered increasing attentions based on the knowledge that cancer cells rely on telomerase activity to sustain their growth, whereas normal cells do not [2,1,2,3,4]. 6-Thio-2'-deoxyguanosine (6-Thio-dG, also known as THIO), a telomerase substrate precursor nucleoside analog [2], is considered to be a highly promising tumor-specific therapeutic agent [5,1,2,3,4,5,6,7,8]. THIO induces telomeric DNA damage via telomerase activity -dependent incorporation, in a telomerase activity-dependent manner, into de novo synthesized telomeres [9]. This mechanism leads to rapid tumor shrinkage or growth arrest in numerous tumor-derived xenograft models, accompanied by minimal side effects [5], [6]. The most significant advantage of this telomere-targeted therapy over direct telomerase inhibitors is that THIO exhibits no substantial delay in its tumor-killing effect. It acts independently of the initial telomere length and directly generates unstable telomeres [10]. Currently, the antitumor efficacy of THIO has been investigated in various cancer types, including non-small cell lung cancer, melanoma, colon cancer, and childhood brain tumors [8].
Immune “hot” tumors are characterized by high immunogenicity, intense immune cell infiltration, and a pro-inflammatory tumor microenvironment (TME). These features render them more susceptible to the stimulation of antitumor immune responses and more responsive to immunotherapies. In contrast, immune "cold" ICD tumors display the opposite characteristics. Consequently, converting an immune "cold" TME into an immune "hot" TME is pivotal for the successful implementation of tumor immunotherapy. Recent evidence has shown that THIO enhances the antigen cross-presentation capacity of dendritic cells (DCs). Its therapeutic effect is correlated with CD8 + T-cells and is dependent on the stimulator of interferon genes (STING) pathway [11], suggesting that THIO has the potential to induce Immunogenic cell death (ICD) in tumor cells. ICD, a form of programmed cell death, has been reported to be associated with a better prognosis in tumor treatment. Tumor cells undergoing ICD produce or present immunostimulatory molecules, such as damage-associated molecular patterns (DAMPs), secrete proinflammatory cytokines, and release tumor antigens. These processes promote the migration and maturation of antigen-presenting cells (APCs), the presentation of tumor antigens to T cells, and the activation of antitumor effector T cells [12,1,2,3,4,5,6,7,8,9,10,11,12,13,14]. The common immune mediators generated by ICD cells include adenosine triphosphate (ATP) and high mobility group protein B1 (HMGB1), which are passively released extracellularly to serve primarily as "find me" signals for attracting immune cells; calreticulin (CALR) and heat shock proteins (HSP70/90), which are exposed on the cell surface to act as "eat me" signals for stimulating APC uptake; and proinflammatory cytokines, such as IFN-γ, IL-1β, and IL-17, which are secreted into the immune microenvironment to promote and regulate immune responses [15, 16].
Conventional clinical tumor treatments, such as radiotherapy and certain chemotherapeutic drugs, can induce ICD in tumor cells through multiple mechanisms, in addition to their direct cytotoxic effects [17,1,2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20]. Currently, traditional chemotherapeutic drugs capable of triggering ICD include adriamycin, mitoxantrone, oxaliplatin, among others [21]. However, the complete induction of ICD in tumors to achieve the desired therapeutic outcomes often necessitates the use of high-dose chemotherapeutic drugs. The resulting severe side effects significantly limit the clinical application of ICD induction [22, 23]. Therefore, the search for low-toxicity, highly specific, and efficient ICD inducers is of paramount importance for the development of novel tumor therapeutic strategies that capitalize on ICD induction and leverage its immune properties.
Our previous brief publications reported on THIO's ability to induce ICD in mouse tumor cells [24]. The primary objective of this study was to investigate whether THIO, in combination with either a nanovaccine (self-assembling peptide Q11 -mediated Q11-E744-62 or Q11-M30 nanofiber, which presents tumor antigen E744-62 or M30) or BBV, could yield synergistic antitumor effects in both immune "hot" and "cold" tumor models. Additionally, we aimed to characterize the immunological response patterns elicited by THIO alone or in combination with other therapeutic approaches. This study also provides more detailed insights into the ICD features induced by THIO and its ability to stimulate dendritic cells (DCs) by inducing ICD in both mouse and human tumor cells in vitro. Moreover, our findings suggest that, beyond its role in killing tumor cells and stimulating antitumor immune responses, THIO may have direct effects on myeloid-derived suppressor cells (MDSCs). This is a proof-of-concept study based on mouse tumor models. We further demonstrated THIO's capacity to induce ICD in human tumor cells in vitro, providing additional support for the potential clinical application of THIO, complementing the findings from animal models. This study broadens the understanding of THIO's antitumor effector mechanisms and offers a promising strategy for the development of ICD-induction and TME remodeling -based antitumor immunotherapy (Scheme 1).
Schematic representation of THIO-triggered antitumor immune responses and its synergistic action with a nanovaccine. A Self-assembly process of Q11-E7 and Q11-M30 nanofibers, which serve as vaccines specifically targeting TC-1 and B16 tumors, respectively. B THIO selectively eliminates tumor cells and induces immunogenic cell death (ICD) in tumor cells, thereby generating "find me" and ‘‘eat me’’ immunostimulatory signals, releasing pro-inflammatory molecules, and presenting a wide spectrum of tumor antigens. These events collectively promote the migration, antigen presentation, and maturation of dendritic cells (DCs), subsequently initiating antitumor responses mediated by cytotoxic T lymphocytes (CTLs) and natural killer (NK) cells. Additionally, THIO directly suppresses the functions of myeloid-derived suppressor cells (MDSCs). Treatment with THIO results in the remodeling of the tumor immune microenvironment. This process synergizes with the tumor vaccine, which is designed to evoke antigen-specific effector T cell responses by facilitating the generation, migration, survival, and maintenance of antitumor T cell activity. CTLs cytotoxic T lymphocytes; MDSCs myeloid-derived suppressor cells, NK natural killer cells, ATP adenosine triphosphate, CALR calreticulin, IL-1β interleukin-1, HMGB1 high mobility group protein B1. The scheme was created using BioRender.com
This study broadens the understanding of THIO's antitumor effector mechanisms and offers a promising strategy for the development of ICD-induction and TME remodeling -based antitumor immunotherapy.
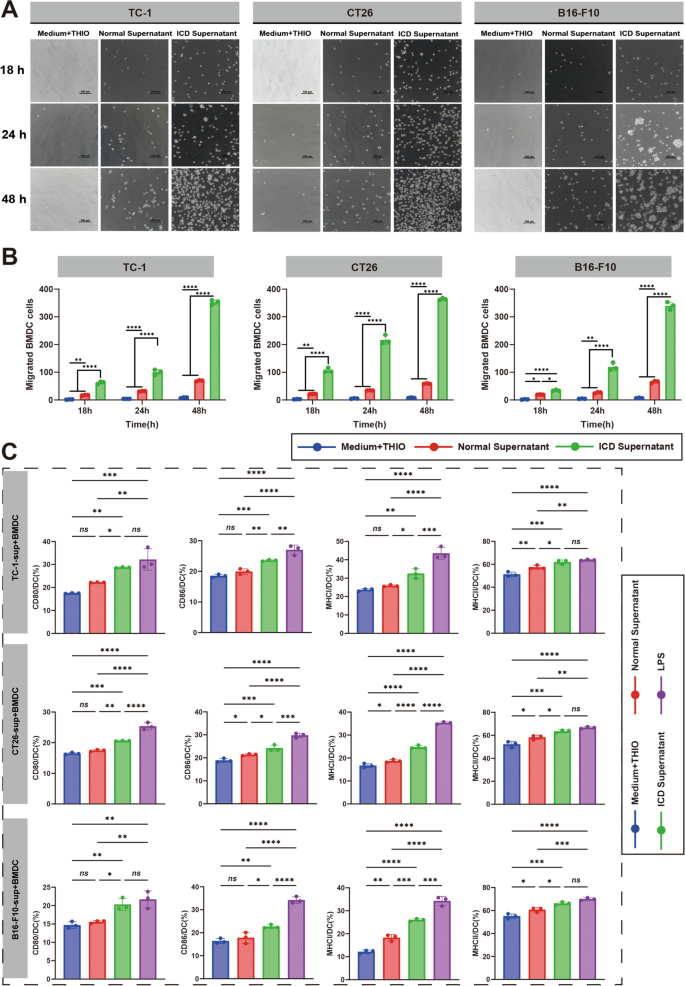
Our previous study has shown that mouse tumor cells CT26, TC-1 and B16-F10 were able to be induced to undergo ICD by the treatment of THIO in vitro [24]. And, the similar ICD features induced in mouse tumor cells were confirmed in THIO -treated human tumor cells Hela and A549, including the morphological changes of the cells getting shrinked and rounded (Figure S1A), decreased cell viability detected by CCK8 assay (Figure S2), increased lactate dehydrogenase (LDH) release (Figure S3B) and increased expression of the immunostimulatory mediators characterizing ICD cells (Figure S3A, S4). The collected supernatants from THIO-treated mouse (Fig. 1A, B) or human (Figure S5) tumor cells were able to stimulate the migration of the mouse myeloid-derived dendritic cells (BMDCs) in a time-dependent manner, while the medium containing THIO showed no effect and the supernatant of normal cultured cells had a slight stimulation. After incubating with the supernatants for 24 h, BMDCs were stained and analyzed with flow cytometry for the expression of maturation markers, and the results showed that the supernatants of THIO-treated mouse or human tumor cells were able to significantly promote the expression of CD80, CD86, MHC I and MHC II molecules in BMDCs, as compared to the normal supernatants and medium containing THIO (Fig. 1C and Figure S6). The results indicated that THIO-treated tumor cells presented the capability of attracting and activating APCs, which is important for triggering an adaptive T cell response.
The culture supernatants of THIO-treated mouse tumor cells stimulates the migration and maturation of BMDCs. A Transwell assay for BMDC migration. BMDC were placed into the upper chamber of 24-well Transwell plates, and then the culture supernatants after THIO treatment of CT26, TC-1 and B16-F10 (i.e., ICD Supernatants), untreated cell culture supernatants (i.e., Normal Supernatants), and THIO-containing blank medium control (i.e., Medium + THIO) were placed into the lower chamber and observed and counted the number of cells in the lower chamber with a light microscope at different time points. B Statistical analyses on BMDC migration at different times (n = 3). C Statistical analyses with flow cytometry on BMDC maturation stimulated by the culture supernatants of THIO-treated tumor cells. Maturation markers CD80, CD86, MHC I and MHC II were detected (n = 3). Data are shown as the mean ± standard deviation (SD), repeat experiment 2–3 times independently. Statistical significance was calculated via one-way analysis of variance (ANOVA) followed by Tukey's multiple comparisons. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001; ns, not significant
In order to confirm the killing effect of THIO in mouse tumors and learn the characteristics of possible immune responses, a tumor model was first established by subcutaneously transplanting mouse colonic carcinoma CT26 cells, and then administrations of THIO at a dose of 2 mg/kg were given every other day intraperitoneally (Fig. 2, 3), intravenously (Figure S7, S8), or intratumorally (Figure S9, S10) respectively when the tumors grew to a diameter of 3–5 mm (Fig. 2A, S7A and S9A). The volume of tumor was monitored continuously every other day, and the results showed that the tumor growth was significantly inhibited by THIO treatment (Fig. 2C, S7C and S9C), which was supported by the results of tumor size (Fig. 2B, S7B and S9B) and tumor weight (Fig. 2D, S7D and S9D). Correspondingly, splenomegaly was suppressed significantly (Fig. 2E, S7E and S9E). MDSCs and splenomegaly During tumor progression, the amplification and accumulation of MDSCs in the spleen is a major driver of splenomegaly [25]. Effective therapies targeting MDSCs (e.g., gemcitabine) reduce splenomegaly in tumor models [26]. Anti-tumor therapies that enhance effector T-cell responses and reduce immunosuppressive cells lead to changes in splenic composition that reduce spleen size [9, 25].
Intraperitoneal treatment of THIO inhibits the growth of subcutaneous CT26 tumors in mice. A Schematic of the treatment schedule: tumor bearing mice received intraperitoneal THIO at 2 mg/kg every other day for a total of five doses. B Representative photographs of excised tumors; dashed circles indicate complete tumor regression. C Tumor growth curves. D Tumor weight summary (n = 8). E Spleen weight summary (n = 8). The mouse experiments were performed in two independent replicates. Data are shown as mean ± standard deviation (SD). Statistical significance for tumor growth curve was calculated by two-way analysis of variance (ANOVA). The comparison between PBS and THIO was conducted by unpaired Student’s t-test. ****p < 0.0001; ns, not significant. Figure A created with BioRender.com
Intraperitoneal treatment of THIO promotes anti-tumor immune response and inhibits MDSCs production. A In the spleen, ELISPOT analyzed the spot statistics of IFN-γ secretion by lymphocytes (n = 3), and flow cytometry analyzed the ratio of MDSCs (CD11b+Gr-1+) and CTL (CD3+CD8+IFN-γ+) in the spleen (n = 5). B In lymph nodes, ELISPOT analyzed the spot statistic of IFN-γ secretion by lymphocytes (n = 3) and flow cytometry analyzed the proportion of MDSCs (CD11b+Gr-1+) and CTL (CD3+CD8+IFN-γ+) in lymph nodes (n = 3). C Representative immunofluorescence images of CTL (blue: nucleus, red: CD8, green: IFN-γ) and MDSCs (blue: nucleus, red: Gr-1, green: CD11b) in tumor tissues. Data are shown as mean ± standard deviation (SD). The comparison between PBS and THIO was conducted by unpaired Student’s t-test. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001
Based on the findings in vitro that THIO treatment caused ICD in tumor cells, the key immune responses were investigated in THIO-treated mice. At the endpoint of the experiment, mouse spleens and lymph nodes were isolated and the lymphocytes were isolated, stained and analyzed with ELISPOT and flow cytometry. ELISPOT results showed that the level of IFN-γ-secreting lymphocytes in spleens and lymph nodes of THIO-treated mouse was significantly increased (Fig. 3, S8 and S10). Further, flow cytometry analyses showed that the level of CTLs in the THIO-treated mice was significantly higher and the level of MDSCs was significantly lower (Fig. 3, S8 and S10), as compared to the mice receiving PBS as a control. Immunofluorescence staining was also performed on tumor tissues isolated and obtained at the end of the experiment, and the same results were obtained (Fig. 3C, S8C and S10C). The results showed that THIO treatment effectively suppressed tumor growth, and more interestingly, it was found that THIO successfully regulated anti-tumor immune responses, which may imply the role of ICD induction in vivo.
Similar to CT26 tumor, our previous study have found that THIO treatment significantly suppressed tumor growth in TC-1 tumor -bearing mice [24]. In this study, we were interested in further examining the information about the cellular immune responses related to THIO treatment in TC-1 tumor. The tumor cells were subcutaneously inoculated on the right side of mice to establish tumor growth. When the tumor diameter reached to 3–5 mm, mice were intraperitoneally injected with THIO (2 mg/kg, 100 µL) every other day. The spleens, tumor tissues and lymph nodes of mice were isolated, and the lymphocytes were obtained after two or five THIO administration respectively. CTLs, MDSCs, NK and Th1 cells, were analyzed by flow cytometry (Fig. 4A). The results showed that, both in spleen and tumor tissues, the levels of IFN-γ+CD8+ CD3+CTLs, IFN-γ+CD3+CD4+ Th1 cells, and CD3−NK1.1+ NK cells were significantly higher and the level of CD11b+Gr-1+ MDSCs were significantly lower in THIO-treated tumor-bearing mice, in comparison with the control group receiving PBS (Fig. 4B, 4C). However, in the lymph nodes, only the level of MDSCs underwent a significant reduction, the changes in the level of NK cells and CTLs were not significant enough (Figure S11). The results together with those found in CT26-bearing mice indicated that THIO successfully regulated TME and systemic antitumor immune responses whether in “immune hot” or “immune cold” tumors while it exerted a direct killing on tumor cells.
THIO treatment improves anti-tumor immune responses and remodels the tumor microenvironment. A Schematic representation of the response of important immune cells after THIO treatment. B Flow cytometry analysis of lymphocytes isolated from mouse spleen (B, top) (n = 3) and tumor tissues (B, bottom) (n = 3) after two administrations of THIO, including CTLs (CD3+CD8+IFN-γ+), NK (CD3−NK1.1+), MDSCs (CD11b+Gr-1+), and Th1 (CD3+CD4+IFN-γ+). (C) Flow cytometry analysis of lymphocytes isolated from mouse spleen (C, top) (n = 3) and tumor tissues (C, bottom) (n = 3) after five administrations, including CTLs (CD3+CD8+IFN-γ+), NK (CD3−NK1.1+), MDSCs (CD11b+Gr-1+) and Th1 (CD3+CD4+IFN-γ+). Each treatment group contained five mice, and the experiment was repeated independently twice. Data are shown as mean ± standard deviation (SD). Statistical significance was calculated by unpaired Student’s t-test. *p < 0.05, ** p < 0.01, ***p < 0.001, ****p < 0.0001; ns, not significant. Figure A created with BioRender.com
MDSCs are key immunosuppressive cells that play an important role in promoting the formation and maintenance of an immunosuppressive and immune escape tumor microenvironment. Based on the finding that the response of MDSCs was significantly suppressed in spleen and tumor tissues of THIO-treated mice, we further investigated whether THIO might act directly on MDSCs. Bone marrow precursor cells were isolated from mouse bone marrow, and then induced by adding GM-CSF, IL-6, and PMA to obtain MDSCs for the subsequent experiments (Fig. 5A). The purity of the obtained MDSCs was identified by flow cytometry to be above 94% (Figure S12A). The CCK8 method was used to detect changes in the viability of MDSCs after treatment with increasing concentrations of THIO. The viability of MDSCs gradually decreased with increasing THIO concentration after 48 and 96 h of treatment (Fig. 5B). In Order to test the effect of THIO on MDSCs differentiation, after isolation to obtain bone marrow precursor cells, the addition of GM-CSF and IL-6 along with different concentrations of THIO together induced the differentiation of MDSCs, and the results showed that THIO treatment significantly inhibited the differentiation of MDSCs (Fig. 5C), and the inhibitory effect became more pronounced with extended stimulation time. And the gating strategy of the flow cytometry (Fig. 5D).
Direct inhibitory effect of THIO on MDSCs. A Schematic representation of MDSCs obtained by isolated stimulation from the bone marrow of hind leg tibia and femur of 8–10 week-old male C57 mice. B Viability of MDSCs after treatment with varying concentrations of THIO for 48 h and 96 h, assessed by the CCK‑8 assay (n = 3). C Flow cytometric analysis of the effect of different THIO concentrations, applied to bone marrow precursor cells for 48 h and 96 h, on subsequent MDSCs differentiation (n = 3). D Flow cytometry gating strategy for identifying MDSCs derived from bone marrow precursor cells. Data are shown as the mean ± standard deviation (SD). Representative results from 2–3 independent experiments. Statistical significance was calculated via one-way analysis of variance (ANOVA) followed by Tukey’s multiple comparisons. ***p < 0.001, ****p < 0.0001; ns, not significant. Figure A created with BioRender.com
It was reported that bacterial membrane vesicles can interfere with the growth of implanted tumor in mice through intravenous administration, due to its tumor -targeting ability and immunoregulative effects in TME [27, 28]. It was speculated that increasing the TME remodeling with BBV may further improve the effectiveness of THIO treatment. In this study tested the efficacy of the combination of THIO and BBV in the treatment of tumors in TC-1 tumor-bearing mice. Ten days after inoculation with TC-1 tumors, hormonal mice were injected intraperitoneally with 2 mg/kg of THIO, 10 ng of BBV, or a combination of THIO and BBV, every other day for a total of 5 injections (Fig. 6A). The dynamic monitoring of tumor volume showed that the mice received the combination of THIO and BBV or THIO alone had a more pronounced inhibitory effect on tumor growth as compared to the tumor-bearing mice receiving PBS or BBV alone, and the combination produced the most obvious effect (Fig. 6C). The results were well supported by checking the size (Fig. 6B) and weight (Fig. 6D) of the tumor masses collected at the endpoint of the experiment. Corresponding to tumor suppression, the splenomegaly was significantly reduced in the mice receiving the combination of THIO and BBV or THIO in comparison with the mice receiving PBS or BBV (Fig. 6E).
Antitumor immune responses and effects of THIO treatment in combination with BBV against an established TC-1 tumor model. A Flowchart showing the treatment of THIO in combination with BBV. B Pictures of collected tumor masses (n = 8). C Growth curve of tumor (n = 8). D Tumor weight (n = 8). E Flow cytometry analysis of the proportion of MDSCs and CTLs in splenocytes (n = 5), with MDSCs (CD11b+Gr-1+) on the left and CTLs (CD3+CD8+IFN-γ+) on the right. G ELISPOT analysis of IFN-γ-secreting lymphocytes (n = 3). Left panel, statistical graph; right panel, representative images. Data are shown as mean ± standard deviation (SD). The mouse experiments were performed in two independent replicates. Statistical significance was calculated by two-way analysis of variance (ANOVA) and via one-way analysis of variance (ANOVA) followed by Tukey’s multiple comparisons. *p < 0.05, ** p < 0.01, ***p < 0.001, ****p < 0.0001, ns, not significant. Figure A created with BioRender.com
The lymphocytes were isolated from spleen tissues, and the level of IFN-γ-secreting T cells was detected by ELISPOT and the important antitumor effector cells CTLs and immune suppressive cells MDSCs were analyzed by flow cytometry. The results showed that the responses of IFN-γ-secreting splenocytes was significantly increased in THIO + BBV and THIO groups in comparison with PBS and BBV groups (Fig. 6G). In addition, THIO + BBV group more significantly increased the level of CTLs in the splenocytes and decreased the level of MDSCs cells (Fig. 6F) than THIO group, as compared to PBS and BBV groups. It implied that THIO might cooperate with BBVs to obtain an enhanced antitumor effect.
Self-assembling peptide Q11-mediated nanofiber carrying HPV-specific antigen E744-62 has proven to be effective for eliciting anti-tumor responses in our previous publications [29, 30]. It is interesting to investigate whether THIO treatment and a tumor vaccine immunization will hold a potential to overcome tumor immunosuppression and escaping and trigger synergistic anti-tumor effects. In this study, when the tumors grew to a diameter of 3–5 mm, the tumor-bearing mice were randomly divided into 4 groups receiving respectively PBS, Q11-E744-62 nanofibers, THIO, and THIO + Q11-E744-62 (Fig. 7A). THIO (2 mg/kg, 100 µL) was administered intraperitoneally every other day starting from day 11 for a total of 5 times, and Q11-E744-62 (10 nmol/agent, 100 µL) was administered by subcutaneous injection three times with a 5 days’ interval. The combination of THIO treatment and Q11-E744-62 immunization more significantly inhibited the growth of the established tumor than Q11-E744-62 immunization or THIO treatment, in comparison with PBS treatment (Fig. 7B). The results of tumor weight and size at experimental endpoint were well consistent with that of tumor growth curves (Fig. 7C and 7D). Correspondingly, the weight of spleen in THIO + Q11-E744-62 group was significantly lighter than those in the Q11-E744-62 and THIO groups, and all of the groups presented lighter spleen as compared to PBS group (Fig. 7E). The lymphocytes in spleen were isolated and analyzed by ELISPOT (Fig. 7G) and flow cytometry. The results revealed that the level of IFN-γ+ in CD8+ T cells was significantly higher and the response of MDSCs was significantly lower in the mice receiving THIO + Q11-E744-62, comparing with the other groups (Fig. 7F, left). And, the level of IFN-γ-secreting splenocytes was also significantly enhanced in THIO + Q11-E744-62 group (Fig. 7F, right). The above results indicated that the combination treatment of THIO and Q11-E744-62 produced a more robust antitumor immune responses than either monotherapy, which hints a possible synergistic effect of tumor killing and TME regulation by THIO and effector T cell generation and responses elicited by Q11-E744-62.
Antitumor immune responses and effects of THIO in combination with a nanovaccine Q11-E744-62 in an established TC-1 tumor model. A Flowchart showing the treatment of THIO and Q11-E744-62 nanofibers. B Dynamic surveillance of tumor volume. C Pictures of the collected tumor masses (n = 5). D Tumor weight (n = 5). E Spleen weight (n = 5). F Flowcytometry analyses on the ratios of CTLs (CD3+CD8+IFN-γ+) and MDSCs (CD11b+Gr-1+) in splenocytes (n = 3). G ELISPOT analysis of IFN-γ-secreting lymphocytes in spleen. Left, representative images; right, statistical graph (n = 3). Data are shown as mean ± standard deviation (SD). The mouse experiments were performed in two independent replicates. Statistical significance was calculated by one-way ANOVA. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001; ns, not significant. Figure A created with BioRender.com
Considering TC-1 tumor is kind of an “immune hot” tumor [31, 32], in this study, an “immune cold” tumor model of subcutaneously transplanted B16-F10 melanoma [32,1,2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34] was employed. When the tumor has fully established, the mice were randomly divided into 4 groups and received the treatment of PBS, Q11-M30, THIO, and THIO + Q11-M30 respectively (Fig. 8A). THIO (2 mg/kg) was administered every other day starting on day 9 for a total of 5 doses, and 20 µg Q11-M30 which is a nanofiber carrying a B16 -specific antigen peptide M30, was administered by subcutaneous injection three times with a 5 days’ interval on day 9. The immunization of Q11-M30 nanofibers and the treatment with THIO significantly suppressed the growth of implanted B16 tumor as compared to the administration of PBS in tumor-bearing mice, meanwhile it was noted that the combination of Q11-M30 nanofiber immunization and THIO treatment produced a more significant antitumor effect than either monotherapy (Fig. 8B and 8C). Further investigation on the responses of CTLs and MDSCs indicated that all the treatment groups presented a significantly increased CTLs response in spleen (Fig. 8D, right) and decreased MDSCs levels in both spleen and tumor (Fig. 8D, left; 8E), while the combination of Q11-M30 immunization and THIO treatment present the most significant effects. The results indicated that even in an event of treating “immune cold” tumor, the combined treatment of THIO and Q11-M30 could cooperate to effectively regulate the immune microenvironment, enhance the antitumor immune responses, and produce more robust antitumor effects than either monotherapy.
THIO in combination with the nanovaccine Q11-M30 elicits effective antitumor immune responses and effects in an established “immune cold” tumor model of B16-F10 melanoma. A Flowchart showing the treatment of THIO and Q11-M30 nanofibers. B Dynamic monitoring of tumor growth (n = 6). C Pictures of collected tumor masses (n = 6). D Flowcytometry analyses on the ratios of CTLs (CD3+CD8+IFN-γ+) and MDSCs (CD11b+Gr-1+) in splenocytes (n = 3). E Flowcytometry analyses on the ratios of MDSCs (CD11b+Gr-1+) in tumor (n = 3). Data are shown as mean ± standard deviation (SD). The mouse experiments were performed in two independent replicates. Statistical significance was calculated by one-way ANOVA. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001; ns, not significant. Figure A created with BioRender.com
THIO is a highly promising telomere-targeting agent with substantial potential for clinical application. It selectively acts on telomerase-positive tumor cells, thereby minimizing damage to normal cells in theory, as supported by previous studies [35, 36]. Beyond its direct cytotoxic effect on tumor cells, emerging evidence indicates that the therapeutic efficacy of THIO may rely on CD8 + T cells, mediated by genome damage and activation of the STING pathway [11]. Our prior research demonstrated that THIO induced cell death in mouse melanoma B16-F10, colon cancer CT26, and cervical cancer—associated tumor TC-1 cells. The characteristics of cell death, including the kinetics of morphological alterations, cell viability, and LDH release, were found to be contingent upon cell type, drug dosage, and treatment duration [24]. Notably, previous investigations revealed that mouse tumor cells treated with THIO exhibited classic hallmarks of ICD. These features included the release of immune-stimulating "find me" signals such as ATP and HMGB1, the secretion of the proinflammatory cytokine IL-1β, and the surface exposure of the "eat me" signal molecule CALR [37,1,2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40]. In this study, we further confirmed that the supernatants of THIO-treated mouse tumor cells promoted the migration, antigen presentation, and maturation of DCs. Additionally, THIO was shown to induce ICD in vitro in human tumor cell lines, such as cervical cancer HeLa, laryngeal carcinoma Hep2, and lung cancer A549 cells.
Inspired by these findings, we aimed to comprehensively investigate the therapeutic effects and immune characteristics of THIO in tumor models. In this study, three distinct tumor cell lines were selected to represent tumors with varying TME characteristics and immune response profiles, thereby enabling us to assess the efficacy of THIO-based therapeutic strategies in eliciting robust antitumor effects and immune responses. The CT26 mouse colorectal cancer cell line is frequently employed to model solid tumors and study immune responses within "moderately immunogenic" environments [41]. The TC-1 lung epithelial cell line, transformed with HPV-16 E6/E7 and Ras oncogenes, serves as a representative model for "immune hot" tumors, characterized by high immunogenicity and extensive T-cell infiltration in the TME [42], making it an ideal choice for investigating antitumor immunity and vaccine efficacy. Conversely, the B16-F10 melanoma cell line is widely recognized as an "immune cold" tumor model, attributed to its low immunogenicity and potent immune suppression mechanisms [43].
First, we demonstrated that THIO significantly inhibited the growth of subcutaneously transplanted CT26 tumors, regardless of whether it was administered systemically (intraperitoneally or intravenously) or locally (intratumorally). With the notable suppression of tumor growth by THIO treatment, the response of antitumor effector T cells was augmented, while the presence of pro-tumor immunosuppressive MDSCs was significantly reduced in both the spleen and the local tumor tissue. These results indicated that the tumor-killing effects of THIO promoted antitumor immunity in tumor-bearing mice, which was consistent with the previously observed ability of THIO to induce ICD in tumor cells during in vitro experiments. Using another tumor model of TC-1-bearing mice, we confirmed that THIO effectively suppressed tumor growth. This suppression was accompanied by enhanced responses of CTL, Th1 cells and NK cells, as well as a decreased MDSCs response in both the spleen and the tumor. The remodeling of the tumor microenvironment into an antitumor state and the significant reduction in MDSCs levels suggested that, in addition to indirectly promoting antitumor effector cell responses, THIO might have a direct effect on MDSCs. This hypothesis was supported by in vitro experimental results showing that THIO treatment reduced the viability and suppressed the differentiation of MDSCs. However, other cell populations within the tumor microenvironment can also profoundly influence antitumor immune responses and tumor growth. For example, regulatory T cells (Tregs), tumor-associated macrophages (TAMs), and exhausted CD8+ T cells are known to play crucial roles in promoting tumor progression and immune evasion [44, 45]. It is plausible that THIO treatment may affect these cell populations in addition to MDSCs. Therefore, comprehensively evaluating the potential direct or indirect effects of THIO on various cell populations within the tumor microenvironment is essential for a more complete understanding of its immunomodulatory mechanisms. Considering THIO's specific tumor-killing effect, its ability to induce ICD in tumor cells, and its direct suppression of MDSCs viability and proliferation—all of which contribute to the stimulation of antitumor immune responses and remodeling of the tumor microenvironment—THIO is considered a promising candidate for clinical tumor treatment. Additionally, we hypothesized that combining THIO with other therapeutic approaches could yield synergistic effects and achieve more optimal therapeutic outcomes. Previous reports have shown that bacterial outer membrane vesicles possess the potential to target tumor tissue and stimulate antitumor responses [46,1,2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48]. Our previous study also demonstrated that bacterial biomimetic vesicles could repolarize tumor-associated macrophages to an antitumor phenotype and remodel the tumor microenvironment [49]. In the present study, we explored the strategy of combining THIO with BBV to enhance tumor treatment efficacy by strengthening immune regulation in the TME and stimulating in situ antitumor immune responses. The results showed that, in TC-1 tumor-bearing mice, the THIO + BBV combination group exhibited more pronounced antitumor immune responses and tumor suppression effects compared with the THIO or BBV monotherapy groups. Although the combination group showed an increase in CTLs and a decrease in MDSCs, no significant difference in tumor volume was observed between the THIO and THIO + BBV groups. The lack of statistical significance was likely attributable to individual experimental variability within the THIO + BBV group. Specifically, two mice in the THIO + BBV group had unusually large tumor volumes, while five of the remaining six mice had significantly smaller tumor volumes compared with the THIO group, and one mouse even experienced complete tumor regression. The presence of these two outlier mice increased the overall variance within the THIO + BBV group, resulting in a large standard deviation that obscured the potentially significant difference between the THIO and THIO + BBV groups.
Tumor vaccines represent a highly attractive and promising immunotherapeutic strategy for cancer treatment. However, they have generally failed to achieve substantial clinical efficacy in practical applications. The immunosuppressive TME and the immune escape and suppression mechanisms developed by tumors are the primary factors contributing to this disappointing clinical outcome [50]. THIO treatment promotes anti-tumor cellular immunity in situ tumors and remodels the TME. Nevertheless, although whole tumor cells provide a broad spectrum of antigens, their immunogenicity is typically weak. In contrast, tumor vaccines are designed to target specific tumor antigens and have the potential to overcome immune suppression and elicit antigen-specific responses. However, the accumulation, survival, and maintenance of effector cell activity in the TME, which are stimulated by vaccines, are restricted by the immunosuppressive cellular and molecular factors within the TME. Therefore, we hypothesized that the combined treatment of THIO administration and tumor vaccination would yield a synergistic or additive effect due to their complementary mechanisms of action. In a TC-1 tumor graft model, we demonstrated that the combination of THIO treatment and the nanovaccine Q11-E744-62—a self-assembled nanofiber composed of Q11 peptide carrying an important HPV 16 Th1/CTL fusion epitope—elicited more robust antitumor immunity and tumor suppression compared to either monotherapy. Considering that the TC-1 tumor is classified as an immune "hot" tumor, which is more amenable to control than immune "cold" tumors, we further utilized a B16-F10 melanoma model to validate the efficacy of the combined treatment strategy. The results showed that the combination of THIO and the nanofiber vaccine Q11-M30 indeed induced more significant antitumor effects.
The results of the phase II THIO-101 trial (NCT05208944), presented at the Society for Immunotherapy of Cancer (SITC) Annual Meeting 2024, revealed that the telomere-targeted drug THIO, when combined with cemiplimab, exhibited durable activity in checkpoint inhibitor-resistant patients with advanced non-small-cell lung cancer (NSCLC) [51]. This combination therapy achieved a higher disease control rate (DCR) and survival benefit. Specifically, treatment with a 180 mg dose of THIO resulted in a 38% objective remission rate (ORR), outperforming standard therapies in a heavily pretreated patient population [52]. However, due to the limited sample size, further large-scale studies are imperative to validate the efficacy and safety of this therapeutic approach. Investigating the potential synergistic effects of THIO with checkpoint inhibitors will be a key focus of our future research.
This study has demonstrated that THIO can ICD in both human and mouse tumor cells. When used alone, THIO significantly inhibited the growth of implanted immune "hot" and "cold" tumors in murine cancer models. Moreover, combining THIO with BBV or nanofiber vaccines more effectively stimulated antitumor immune responses and produced potent antitumor effects. THIO also appears to directly target MDSCs, effectively remodeling the TME. The findings strongly suggest that THIO is a promising candidate for clinical tumor treatment, and its combination with other approaches, such as nanovaccines, can achieve more favorable antitumor effects, providing a novel strategy to enhance the clinical efficacy of tumor immunotherapy. However, this study has several limitations. For example, the mechanisms by which THIO acts on MDSCs and its potential impacts on other key immune cell populations remain unexplored. These aspects warrant in-depth investigation in future research.
Female BALB/c mice (16–18 g, 6–8 weeks old), male and female C57BL/6 mice (16–18 g, 6–8 weeks old) were provided by the Department of Small Animal Experimentation, Institute of Medical Biology, Chinese Academy of Medical Sciences (CAMS), and housed in their SPF-grade animal house. All mice had free access to food and water, and were provided with artificial light through a 12 h light/12 h dark cycle. All animal handling was in accordance with ethical standards and approved by the Ethics Committee for Animal Care and Welfare of the Institute of Medical Biology, CAMS (Ethics No. DWSP202004026). TC-1 cell, which is C57BL/C (H-2b) lung epithelial cell co-transfected with HPV16 E6/E7 and ras genes, was supplied by the Tumor Cell Bank, CAMS. Murine colonic tumor CT26 and melanoma B16-F10, and human cervical cancer Hela, laryngeal carcinoma Hep-2 and lung cancer A549 cells were supplied by the American Type Culture Collection (ATCC). All cells were cultured in Roswell Park Memorial Institute (RPMI) 1640 medium (Gibco) or DMEM (Gibco), which contains 10% FBS (Gibco) and 1% penicillin–streptomycin (BI) at 37 °C in 5% CO2.
The viability of tumor cells was assayed using the CCK8 kit (Shanghai Huilong Biotechnology) according to the manufacturer's instructions. Briefly, cells were seeded on 96-well plates as 100 µL/well at a density of 3 × 105 cells/mL, and incubated at 37 ℃ in a 5% CO2 incubator for 12 h. Cells were treated with THIO at different concentrations (0, 10, 20, 40, 60, 80 and 100 µmol/L) for a certain period of time. The prepared CCK8 assay solution was added into the culture plate and incubated at 37 ℃ in 5% CO2 incubator for 1.5 h. The absorbance value at 450 nm was measured by enzyme marker ad used for cell viability calculation, according to the formula: Cell survival rate = [(As–Ab)/(Ac–Ab)] × 100% (As: drug-treated cell; Ab: blank; Ac: cell control).
LDH release in cell culture supernatants were determined using the LDH kit (Beyotime) according to the manufacturer's instructions. Briefly, cells were seeded on 48-well plates as 300 µL/well at a density of 3 × 105 cells/mL and incubated for 12 h at 37 °C in a 5% CO2 incubator. After the cells were treated with different concentrations of THIO (0, 10, 20, 40, 60, 80 and 100 µmol/L) for a certain period of time, the culture supernatant was collected. The supernatant was loaded into a new 96-well plate having been added with the assay working solution at 120 µL/well. The plates were incubated at room temperature for 30 min in dark, and the absorbance values of the supernatant samples were measured at 490 nm using an enzyme marker for calculating LDH release, according to the following formula: LDH release (%) = [(A2–A0)–(A1–A0)]/[(A3–A0)–(A1–A0)] × 100%. (A0: blank; A1: cell control; A2: drug-treated cells; A3: maximum release of lysed untreated cells).
ATP release was measured with an ATP detection kit (Beyotime), according to the manufacturer's instructions and our previous report [53].
The release of HMGB1 was analyzed using a HMGB1 detection kit purchased from Wuhan Yunclone Technology Co. Ltd., according to the manufacturer's instructions.
The translocation of CALR on the cell surface was detected by immunofluorescence. Briefly, cells were inoculated into 15 mm glass bottom dishes at a density of 5 × 105 cells/mL, and incubated at 37 ℃ for 12 h in a 5% CO2 incubator. After a treatment of 20 μmol/L THIO for 48 h, the cells were fixed for 30 min in 4% paraformaldehyde (Biosharp) at room temperature for 30 min. After blocking with 5% BSA, mouse anti-CALR monoclonal antibody (Santa Cruz biotechnology, USA) at 1:250 was added, followed by an incubation of HRP-labeled goat anti-mouse IgG H&L (Alexa FluorⓇ647, ab150115) (1:800) for 1 h at room temperature in dark. The nuclei were stained dropwise with DAPI (Abcam, ab104139), and then the stained slides were mounted and photographed through a microscope (Nikon ECLIPSE C1).
The level of IL-1β was measured with enzyme-linked immunosorbent assay (ELISA) using an IL-1β antibody pairs (ab241673) purchased from Abcam, UK, according to the manufacturer's instructions. Briefly, the plate was coated with capture antibody overnight at 4 °C, and then blocked with 2% BSA at 37 °C, followed by sequential incubation with the samples, biotinylated detection antibody and HRP-avidin at room temperature for 2 h. And then the substrate was added to develop the reaction and the absorbance at 450 nm was recorded by an ELISA reader.
Briefly, the femur and tibia were collected from 6 to 10 week-old mice and the bone marrow cavity was repeatedly rinsed. The bone marrow fluid was passed through a 70-μm sieve (Bioscience), and the cells were collected by centrifugation at room temperature. After lysing red blood cells, the cells were washed and resuspended in the complete medium of RPMI-1640 at a concentration of 2 × 106 cells/mL, which is isolated bone marrow cells. 10 mL of the cell suspension was added to the cell culture dish, and incubated with an addition of GM-CSF (20 ng/mL) at 37 ℃ in a 5% CO2 incubator for a total of 10 days. The semi-adherent cells were collected by centrifugation to obtain BMDCs.
BMDCs were inoculated into 96-well plates at a density of 1 × 106/100 μL per well. The supernatant of tumor cells treated with THIO for 24 h and 48 h were added to the cultured BMDCs, with normal supernatant used as a negative and 2 μg/mL LPS used as a positive control. The cells were incubated for 24 h, and then collected. The cells were washed and stained with flow cytometry antibodies PE anti-mouse CD11c and FITC anti-mouse CD80, CD86, MHC I and MHC II (Biolegend) at room temperature in dark for 30 min, and then detected by flow cytometer. The data were analyzed using flowjo software.
BMDCs were inoculated into the upper chamber of a 24-well Transwell plate (Corning) with a pore size of 8 μm at a density of 1 × 105/200 μL per well, and 500 μL of the supernatant of tumor cells treated with THIO was added to the lower chamber. Cell counting was performed using an automated cell counter (invitrogen). The plate was then placed in an incubator at 37 ℃ with 5% CO2. After incubating for 18 h, 24 h and 48 h, the cell migration in the lower chamber were photographed by light microscope (Leica), and 3 fields were randomly selected for cell counting and statistical analysis.
Mouse bone marrow cells were first obtained as described above. For inducing MDSCs differentiation, GM-CSF and IL-6 (MedChemExpress) were added to a concentration of 20 ng/mL, and 10 mL of the cells were added to the cell culture dish and incubated at 37 ℃ in a 5% CO2 incubator for 6 days. And then, 0.5 μg/mL of PMA (MedChemExpress) was added, and the cells were incubated for 3 days and collected by centrifugation to obtain MDSCs [54, 55].
The preparation of BBVs was performed by the procedure reported previously [56]. Briefly, the cultured bacterial cells were collected by centrifugation, resuspended with HBSS solution (Servicebio), and then homogenized three times at 4 ℃ under the pressure of 1200 bar in a high-pressure homogenizer. The suspension was centrifuged for 30 min at 4 ℃ and 10,000 g to pellet the bacterial fragment, and the supernatant was centrifuged for 30 min at 4 ℃ and 10,000 g to precipitate the bacterial fragments. The supernatant was further centrifuged at 4 ℃, 40,000 g for 30 min, and the precipitated BBV was resuspended with HBSS. After the protein concentration was determined by the BCA assay, the BBV samples were stored in a freezer at -80 ℃ and used for subsequent experiments.
The preparation of nanofibers was performed by the procedure reported previously [29]. Two fusion peptides Q11-E744-62 (QAEPDRAHYNIVTFCCKCD-SGSG-QQKFQFQFEQQ) and Q11-M30 (PSKPSFQEFVDWENVSPELNSTDQPFL-SGSG-QQKFQFQFEQQ) were synthesized by Sangon Biotech (Shanghai, China). E744-62 is an important HPV-derived Th and CTL fusion epitope in TC-1 cells, and M30 is a MHC II -restricted epitope in B16 cells. The peptides were dissolved respectively with ddH2O to a concentration of 2 mmol/L and left at 4 °C overnight to allow the formation of protofibers, and then diluted fourfold to 0.5 mmol/L with PBS buffer (Servicebio) at pH = 7.4 and left at room temperature for 6 h. This process resulted in the self-assembly of the Q11 based fusion peptides to form nanofibers, which were stored at -20 ℃ for further use.
For CT-26 tumor model, 100 μL of cell suspension at a concentration of 1 × 106 cells/mL was injected subcutaneously into the right side of female BALB/C mice. In the B16-F10 tumor model, 100 μL of cell suspension at a concentration of 1 × 106 cells/mL was injected subcutaneously into the right side of female BALB/C mice. For the TC-1 tumor model, an equal volume of matrix gel was mixed with tumor cells, and then 100 μL of the mixture at a concentration of 1 × 106 cells/mL was injected subcutaneously into the right side of C57BL/6 mice.
Animal experiments were conducted using female mice under specific pathogen-free (SPF) conditions. Different tumor models and treatment regimens were applied as follows:
CT-26 Tumor Model: Once tumors were palpable and reached the target volume, mice were randomly divided into three groups receiving different THIO administration routes: intraperitoneal (i.p.) injection, intravenous (i.v.) injection via the tail vein, and intratumoral (i.t.) injection. THIO was administered every other day at a dose of 2 mg/kg in 100 µL per mouse, for a total of five doses.
TC-1 Tumor Model: Once tumors were palpable and reached the target volume, mice received one of the following treatments: THIO alone (i.p. injection), Q11-E744-62 nanovaccine alone (subcutaneous, s.c. injection), or combination therapy (THIO + Q11-E744-62). THIO was administered every other day at 2 mg/kg in 100 µL per mouse for five doses. Q11-E744-62 was administered every five days at a dose of 10 nmol per mouse in 100 µL per injection.
B16-F10 Tumor Model: Once tumors were palpable and reached the target volume, mice received one of the following treatments: THIO alone (i.p. injection), Q11-M30 nanovaccine alone (s.c. injection), or combination therapy (THIO + Q11-M30). THIO was given every other day at a dose of 2 mg/kg in 100 µL per mouse for five doses. Q11-M30 was administered every five days at a dose of 20 nmol per mouse in 100 µL per injection.
For all experiments, the control groups consisted of mice treated with equivalent volumes of vehicle (PBS) following the same administration schedule as the treatment groups. Tumor volumes were measured every two days using calipers, and calculated with the formula: Tumor volume (mm3) = 0.5 × length × (width).2
Flow cytometry analysis of splenic lymphocytes and tumor infiltrating lymphocytes was performed as described previously [57]. Briefly, spleen was pressed into a 70 μm cell mesh (Becton, Dickinson and Company Falcon, USA) to obtain dispersed splenocytes. For the separation of lymphocytes from tumor tissue, the tumor tissue was diced into a crumb and digested with 1 mg/mL of Collagenase solution (Sigma, 9001-12-1). The digested suspension was passed through a cell mesh. The cells were aliquoted at 1 × 106 in 96-well U-shaped plate (Corning) and stimulated with 5 μg/ mL E749-57 or M30 peptide for 7 h. Then, 5 μg/mL brefeldin A (BioLegend, San Diego, CA, USA) was added to promote intracellular cytokine accumulation. The cell surface was stained with FITC anti-mouse CD3 (100,204), APC anti-mouse CD8 (100,712), APC anti-mouse CD4 (100,412), APC anti-mouse CD11b (101,212), PE anti-mouse Gr-1 (108,408), APC anti-mouse NK1.1 (156,506), Pacific Blue™ anti-mouse CD45 (982,306). For intracellular cytokine staining on CTLs, the cells were fixed/permeabilized, and then PE anti-mouse IFN-γ (505,808) antibody was used. All monoclonal antibodies were purchased from BioLegend, Inc. Finally, the cells were detected using a CytoFLEX flow cytometer (Beckman Coulter) and analyzed using FlowJo (Tree Star) software.
The assay was performed as described previously [8, 53]. Briefly, 3 × 105 cells were inoculated in each well of 96 well plate and stimulated with 5 μg/ mL E749-57 at 37 °C and 5% CO2 for 4 h. Cells treated with PMA or PBS were used as positive or negative controls, respectively. Cells expressing IFN-γ were detected by sequential incubation with biotinylated anti-mIFN-γ antibody, HRP-coupled affinity and substrate. Visualized spots were counted using the ELISPOT reader system (AID), and spots were recorded and statistically analyzed.
GraphPad Prism 9.0 software was used for statistical analysis. All values in the study were expressed as mean ± standard deviation (SD). Two-way ANOVA was used for analyzing tumor growth curve. One-way ANOVA followed by Tukey's multiple comparisons were performed when comparing more than two groups. Comparisons between the two groups were analyzed using the t-test. Significant differences were represented as the follows: ns, not significant, *p < 0.05; **p < 0.01; ***p < 0.001; and ****p < 0.0001.
No datasets were generated or analysed during the current study.
We would like to thank the authors of this work. The figures in this article were created using Adobe Illustrator and BioRender.
This work was financially supported by the National Natural Science Foundation of China (82073371, 82471865, 82203034), the CAMS Initiative for Innovative Medicine (2021-I2M-1-043), Major Project in Basic Research of Yunnan Province (202401BC070009), the Science and Technology Leading Talent Program of Yunnan Province (202405AB350002), and the Science and Technology Project of Yunnan Province (202201AT070238, 202201AS070061).
All animal procedures were approved by the Institutional Animal Care and Use Committee of the Institute of Medical Biology, Chinese Academy of Medical Sciences.
All authors agreed to publish this manuscript.
The authors declare no competing interests.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
Bai, J., Wang, M., Luo, Y. et al. Tumor microenvironment remodeling with a telomere-targeting agent and its cooperative antitumor effects with a nanovaccine. J Nanobiotechnol 23, 429 (2025). https://doi.org/10.1186/s12951-025-03471-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12951-025-03471-2