Journal of Nanobiotechnology volume 23, Article number: 419 (2025) Cite this article
Reactive oxygen species (ROS)-based nanodynamic therapy is emerging as a promising approach for tumor treatment, particularly in eliciting immune responses for tumor immunotherapy. Nevertheless, the complex tumor microenvironment (TME) and the constraints of current sensitizers substantially compromise therapeutic efficacy. To address these challenges, we developed a rationally designed nanoplatform (LP/CuTT) through lipoic acid-modified orchestrated Cu²⁺-coordinated tetracycline-porphyrin self-assembly, to precisely remodel the immunosuppressive TME while potentiating antitumor immunotherapy via a novel photo-enhanced chemodynamic therapy (CDT) strategy. In vitro studies demonstrated that LP/CuTT-mediated photo-enhanced CDT effectively promoted concurrent apoptosis and cuproptosis in B16-F10 melanoma cells, coupled with robust induction of immunogenic cell death (ICD). Mechanistic investigations revealed that LP/CuTT drives macrophage polarization from tumor-promoting M2 to antitumor M1 phenotypes while promoting dendritic cell (DC) maturation, thereby orchestrating potent antitumor immune responses. In vivo evaluations showed preferential tumor accumulation of LP/CuTT, correlating with substantial ROS generation at tumor sites and remarkable therapeutic outcomes. Quantitative assessments further demonstrated elevated M1 macrophage infiltration in both tumor and splenic tissues, accompanied by enhanced CD8+ and CD4+ T cell recruitment. These findings provide key insights into developing orchestrated metal-coordinated nanotherapeutics by repurposing existing therapeutic agents, enabling the design of multifunctional systems that integrate efficient chemodynamic activity, TME remodeling, and immune activation for effective nanodynamic therapy.
With the advancement of nanotechnology, nanodynamic therapies (NDTs), which eliminate cancer cells through reactive oxygen species (ROS) generation, have emerged as promising tumor treatment strategies [1]. NDTs offer distinct advantages including non-invasiveness, minimal side effects, and tumor-specific targeting. Chemodynamic therapy (CDT), photodynamic therapy (PDT), sonodynamic therapy (SDT) are the three principal modalities within NDTs, which can be employed either individually or in synergistic combinations for treating cancer. Recent research has increasingly focused on ROS-induced immunogenic cell death (ICD), highlighting the crucial role of ROS in eliciting antitumor immune responses [2]. The unique immune-activating effects of NDTs make them highly promising for tumor combination immunotherapies. However, the therapeutic efficacy of NDTs remains substantially limited due to the complexity of the tumor microenvironment (TME) and the inherent limitations of current sensitizers [3]. Immunosuppressive factors in the TME, such as tumor-associated macrophages (TAMs), myeloid-derived suppressor cells, and regulatory T cells, significantly hinder the effectiveness of antitumor immune responses mediated by NDTs. Notably, TAMs, a highly plastic immune cell population with both pro- and anti-tumorigenic roles, tend to reprogram toward the M2 pro-cancer phenotype rather than the M1 anti-cancer phenotype in the immunosuppressive TME [4].
CDT is a promising therapeutic modality that utilizes endogenous overexpression of hydrogen peroxide (H2O2) in tumors to generate toxic hydroxyl radicals (·OH) via a Fenton/Fenton-like reaction catalyzed by iron ions [5]. Compared with other ROS-based therapies, CDT offers advantages such as higher catalytic efficiency for ROS generation, less dependence on external stimuli, and deep tissue penetration. To further enhance the immunotherapeutic efficacy of CDT, numerous studies have explored the development of metal-based nanocarriers beyond iron [6]. Among these, copper-based systems are particularly promising, as copper ions can enhance the efficiency of Fenton-like reactions and induce cancer cell death through cuproptosis. Recent studies have demonstrated the potential of cuproptosis in inducing ICD and enhancing immunotherapy [7]. To harness cuproptosis, various copper-based nanoparticles have been developed. Inorganic nanomaterials such as copper oxide and copper peroxide nanodots have demonstrated a powerful killing effect on cancer cells. However, these materials also pose challenges, including potential toxicity to pre-implantation development of mouse embryos and metabolic difficulties in vivo [8]. Copper-based metal-organic frameworks (MOFs), characterized by porous structures and high drug-loading efficiency, have shown favorable outcomes in antitumor studies. However, their synthesis often requires harsh conditions and organic reagents necessitating caution in biological applications [9]. Self-assembly techniques have emerged as a promising approach for constructing copper-based nanoparticles with satisfactory in vivo safety profiles [10]. Nevertheless, ensuring the retention of the inherent advantages of raw materials while enhancing the CDT efficiency of the metal base remains a challenge.
PDT involves the generation of cytotoxic ROS by exposing photosensitizers to specific light wavelengths, which kill cancer cells by inducing structural and componential photo-induced damage or activating immune and inflammatory responses. PDT has shown great potential in inducing ICD and reprogramming TAMs to M1 phenotype [11]. Photo-enhanced CDT can further improve the catalytic activity and treatment controllability. The synergy between PDT and CDT can enhance therapeutic efficacy and overcome multidrug resistance. However, the immunosuppressive TME significantly limits the efficacy of PDT, and the phototoxicity of photosensitizers remains a concern [12].
Self-assembly using metal ions as a bridge to existing photosensitizers [13], natural products [14] and even Chinese herb [15] may yield unexpected therapeutic effects. For example, tetrakis (4-carboxyphenyl) porphyrin (TCPP), a typical photosensitizer, exhibits excellent biocompatibility and effective clearance properties. Its absorption in the red light region enables its application in the treatment of deep-seated tumors [16]. TCPP coupled with copper, iron, or zinc ions have been explored for effective PDT [17]. Tetracycline (Te) has demonstrated anti-tumor and metastasis-inhibiting properties [18]. Combining Te with copper ions has shown potent cytotoxicity against K562 cancer cells [19]. Recent studies indicate that tetracycline antibiotics, such as eravacycline (ERV), can inhibit tumor cell proliferation, promote ROS production, and induce M1 polarization of macrophages [18], suggesting their potential to modulate the immunosuppressive TME. However, research on the anticancer effects of Te remains limited.
Building on these findings, we propose integrating Cu2+, Te, and TCPP to combine the cuproptosis-inducing capability of Cu2+, the ROS-generating and TAM-repolarizing properties of Te, and the PDT activity of TCPP. This multifunctional approach aims to regulate the immunosuppressive TME through multiple pathways, achieving potent photo-enhanced CDT. Using a self-assembly strategy, we synthesized CuTT from Cu2+, Te, and TCPP (Scheme 1) with an average particle size of approximately 150 nm. Polyvinylpyrrolidone (PVP) was employed as both a blocker and stabilizer [20] to enhance the stability of CuTT, resulting in P/CuTT. Finally, P/CuTT was functionalized with lipoic acid (LA) to form LP/CuTT. LA plays an important role in biomaterials due to its amphiphilic molecular structure. The disulfide bonds in LA molecules exhibit sensitivity to both glutathione (GSH) and ROS [21], making them promising candidates for TME-responsive applications. More importantly, Cu2+ in the nanoassembly undergoes reduction to Cu+ by GSH, enabling Cu+ to react with excess H2O2 in tumor cells for generating ·OH, thereby enhancing CDT. In vitro studies demonstrated that LP/CuTT exhibited a strong killing effect on B16-F10 cells under 680 nm laser conditions at 50 µg/mL while showing minimal dark toxicity toward NIH/3T3 cells. LP/CuTT can effectively induce apoptosis and cuproptosis while stimulating immune responses. In vivo experiments revealed that LP/CuTT with excellent tumor targeting effects, enhanced infiltration of CD4+ T cells, CD8+ T cells, and M1 TAMs in both tumor sites and spleen, ultimately achieving significant therapeutic efficacy in a mouse melanoma model. The LP/CuTT construction strategy presented here offers valuable insights for the design of smart drug delivery systems by utilizing commercially available antibiotics and photosensitizers, enabling multifaceted regulation of the immunosuppressive TME to achieve enhanced antitumor effects.
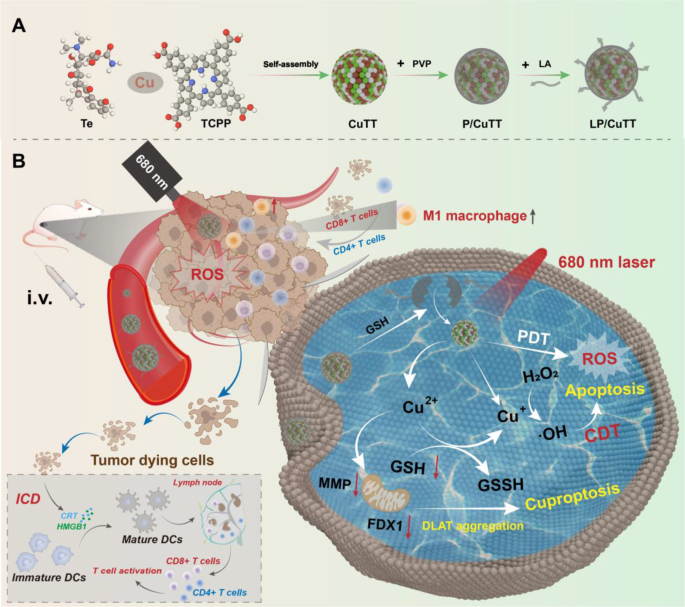
The schematic illustration of the preparation (A) and therapeutic mechanism (B) of LP/CuTT nanoparticles
Te (CAS: 60-54-8, chemical purity ≥ 98%), copper (II) chloride (CuCl2), TCPP, PVP, GSH, H2O2, ammonium molybdate ((NH4)2MoO4), granulocyte-macrophage colony-stimulating factor (GM-CSF) and methylene blue (MB) were got from Aladdin Biochemistry Technology Co., Ltd. (Shanghai, China). LA, lipopolysaccharide (LPS), fetal bovine serum (FBS), and the bicinchoninic acid (BCA) Protein Assay Kit were obtained from Meilun Biotechnology Co., Ltd. (Dalian, China). Indocyanine green (ICG), hydroxyphenyl fluorescein (HPF), 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) was purchased from Sigma-Aldrich (USA). 2’,7’-Dichlorodihydrofluorescein diacetate (DCFH-DA) and Ellman’s Reagent (DTNB) were obtained from Thermo Fisher Scientific (USA). Anti-high mobility group box 1 (HMGB1) mouse monoclonal antibody (PTR2339), anti-calreticulin (CRT) monoclonal antibody, and anti-β-actin mouse polyclonal antibody were purchased from Immunoway Biotechnology (USA). Goat Anti-Rabbit IgG H&L (Alexa Fluor® 488) was obtained from Abcam (Shanghai, China). 2,2,6,6-Tetramethylpiperidine (TEMP) was obtained from Energy Chemical (Shanghai, China). Anti-PDC-E2 (Dihydrolipoamide S-acetyltransferase, DLAT) antibody and recombinant anti-ferredoxin 1 (FDX1) rabbit antibody were purchased from Cohesion Biosciences (Suzhou, China). PE anti-mouse CD206/MMR antibody, FITC anti-mouse CD86 antibody, APC anti-mouse CD80 antibody, FITC anti-mouse CD3 antibody, PerCP anti-mouse CD8 antibody, and APC anti-mouse CD4 antibody were purchased from Elabscience (Wuhan, China). Hoechst 33342, Rhodamine 123, Annexin V-FITC/Propidium iodide (PI) apoptosis detection kit, enhanced chemiluminescence (ECL) substrate kit, and Horseradish peroxidase (HRP)-conjugated secondary antibodies were purchased from Beyotime Biotechnology (Shanghai, China). Urea, Ethylene Diamine Tetraacetic Acid (EDTA), and Tween-20 were purchased from Macklin Biochemical Technology Co., Ltd. (Shanghai, China). Bovine serum albumin (BSA) was obtained from Yancheng Saibao Biotechnology (Jiangsu, China). Mouse interleukin-10 (IL-10) and mouse interleukin-1β (IL-1β) enzyme-linked immunosorbent assay (ELISA) kits were obtained from Solarbio Life Science (Beijing, China). Mouse interleukin-6 (IL-6) and mouse tumor necrosis factor-α (TNF-α) ELISA kits were purchased from MultiSciences (Hangzhou, China). Recombinant interleukin-4 (IL-4) was provided by Novoprotein Scientific Co., Ltd. (Suzhou, China). TUNEL apoptosis detection kit were purchased from YEASEN Biotech (Shanghai, China). Hydroxyphenyl fluorescein (HPF) was obtained from Maokang Biotechnology (Shanghai, China). Hematoxylin and Eosin (H&E) Staining Solution was purchased from Biosharp Life Science (Hefei, China).
The synthesis of the nanoparticles was initiated by mixing Te (1 mg/mL, 1000 µL) with CuCl2 (1 mM, 1000 µL) and then stirring at 0 °C. Subsequently, TCPP (1 mg/mL, 100 µL) was added dropwise to the above solution under stirring conditions to obtain the unmodified nanoparticles CuTT. To prepare P/CuTT, PVP (1 mg/mL, 1000 µL) was introduced into the CuTT suspension. For LP/CuTT synthesis, PVP (1 mg/mL, 100 µL) and LA (1 mg/mL, 900 µL) were added to the CuTT system. All samples were stabilized overnight at 4 °C. Finally, the samples were placed in a 7 kDa dialysis bag and dialyzed for 12 h. The nanoparticles were stored at 4 °C in the dark. Te and TCPP were measured for content using ultraviolet and visible (UV–vis) spectroscopy by a UV–vis spectrophotometer (Shimadzu, Japan). The particle size and zeta potential measurements were performed using dynamic light scattering (DLS) on a Zetasizer Nano ZS (Malvern Instruments, UK). To assess the stability of nanoparticles, CuTT, P/CuTT, and LP/CuTT were suspended in saline, and their particle sizes during a three-day incubation at 4 °C were measured using DLS. The morphologies of the nanoparticles were determined using transmission electron microscopy (TEM, HT7700, Hitachi, Japan). X-ray photoelectron spectroscopy (XPS) measurements were carried out on a Thermo Fisher Scientific ESCALAB 250 A spectrometer.
To investigate the intermolecular forces governing self-assembly, EDTA (20 mM), Tween-20 (20 mM), urea (20 mM), DMSO (10%), and NaCl (0.9%) were introduced to CuTT, P/CuTT, and LP/CuTT systems, and the nanoparticle size variations were monitored.
The degradation of MB was used to determine the generation of ·OH in a H2O2 (1 mM) environment. Cu2+ (50 µM), CuTT, P/CuTT, or LP/CuTT (50 µg/mL) were mixed with MB (2 µg/mL), and then treated with or without a 680 nm laser (1 W/cm2, 2 min). After 10 min of stabilization, the UV–vis spectra were detected by a UV–vis spectrophotometer. Electron spin resonance (ESR) measurement was used to determine the singlet oxygen (1O2) with TEMP as a spin trap to capture the 1O2.
GSH (1 mM, 200 µL) and Cu2+, CuTT, P/CuTT, or LP/CuTT (50 µg/mL, 200 µL) were added into a 1.5 mL centrifuge tube, respectively. After treatment with a 680 nm laser (1 W/cm2, 2 min), the DTNB (0.5 mM, 200 µL) and NaOH (5 µM, 50 µL) were added for further testing. GSH depletion was calculated by detecting the absorption at 412 nm on a microplate reader.
$${\rm{GSH\,consumption}}\left( {\rm{\% }} \right) = \left( {1 - {{{{\rm{E}}_{{\rm{A}}0}} - {{\rm{E}}_{{\rm{A}}1}}} \over {{{\rm{C}}_{{\rm{A}}0}} - {{\rm{C}}_{{\rm{A}}1}}}}} \right) \times 100{\rm{\% }}$$
Where EA0 and EA1 represent the absorbance changes of the experimental group, and CA0 and CA1 represent the absorbance changes of the control group.
For the detection of H2O2, 200 µL of LA (50 µg/mL), CuTT (50 µg/mL), P/CuTT (50 µg/mL), or LP/CuTT (50 µg/mL) was added into a 1.5 mL centrifuge tube containing 200 µL of H2O2 (5 mM) and incubated at 37 °C for 10 min, then (NH4)2MoO4 (200 µL, 10mM) was added, and the absorbance at 405 nm was measured on a microplate reader.
$${{\rm{H}}_2}{{\rm{O}}_2} {\rm{\,consumption}}\left( {\rm{\% }} \right) = \left( {1 - {{{{\rm{E}}_{{\rm{A}}0}} - {{\rm{E}}_{{\rm{A}}1}}} \over {{{\rm{C}}_{{\rm{A}}0}} - {{\rm{C}}_{{\rm{A}}1}}}}} \right) \times 100{\rm{\% }}$$
Where EA0 and EA1 represent the absorbance changes of the experimental group, and CA0 and CA1 represent the absorbance changes of the control group.
The morphological changes of CuTT, P/CuTT, and LP/CuTT before and after exposure to GSH (1 mM) were analyzed by TEM.
Cell lines
B16-F10, NIH/3T3 and RAW 264.7 cell lines were obtained from the Cell Resource Center of Shanghai Institute for Biological Sciences (Chinese Academy of Sciences, Shanghai, China). B16-F10 cells were maintained in Roswell Park Memorial Institute (RPMI)-1640 medium, and NIH/3T3 and RAW 264.7 cells were cultured in Dulbecco’s Modified Eagle Medium (DMEM). All media were supplemented with 10% FBS, 100 U/mL penicillin, and 100 µg/mL streptomycin. Cells were maintained at 37 °C in a humidified atmosphere containing 5% CO2. Female C57 mice (6–8 weeks) from Wushi Experimental Animals Center (Fuzhou, China) were used for dendritic cells (DCs) isolation and induction. Red blood cells were collected from ICR mice.
Cellular uptake and membrane permeability evaluation
B16-F10 cells were seeded in 12-well plates at a density of 1 × 105 cells/well. After 12 h of adherent growth, the cells were treated with CuTT, P/CuTT, or LP/CuTT at a concentration of 50 µg/mL for 2, 4, and 6 h. Subsequently, the cells were harvested and analyzed using flow cytometry (Beckman CytoFLEX, China).
To evaluate membrane permeability, B16-F10 cells were first treated with either saline or LA (50 µg/mL) for 24 h, followed by the addition of PI dye. The uptake of PI dye was measured by flow cytometry at 2, 4, and 6 h.
MTT assay
MTT assay was performed according to the previous report [22]. In brief, B16-F10 or NIH/3T3 cells were inoculated into 96-well plates at a density of 5 × 103 cells per well and cultured for 12 h. Then, different concentrations of materials were added separately, and an MTT assay was performed 24 h later. If laser irradiation is required, it is performed 6 h after drug administration. Laser parameters (wavelength: 680 nm, power density: 1 W/cm2, irradiation time: 2 min).
Detection of intracellular total ROS and ·OH
B16-F10 cells were seeded in a 12-well plate with cell crawling slices. Subsequently, CuTT, P/CuTT, or LP/CuTT (50 µg/mL) was added, and the cells were incubated for 6 h. After that, the cells were incubated with DCFH-DA (10 µM), followed by 680 nm laser (1 W/cm², 2 min) treatment, and finally stained with Hoechst 33342 (10 µM) for another 20 min. The generation of ROS was monitored using a confocal laser scanning microscope (CLSM, Leica TCS SP8, Germany).
For the detection of ·OH, B16-F10 cells were seeded into 12-well plates. The methodology employed for processing the cells is analogous to that described above, except for using HPF (10 µM) probes for staining purposes. After treatment with different materials and laser, the cells were harvested and subjected to flow cytometric analysis.
Detection of intracellular GSH
B16-F10 cells were seeded in 6-well plates at a density of 5 × 10⁵ cells per well. The CuTT, P/CuTT, and LP/CuTT (50 µg/ml) groups were then treated with or without a 680 nm laser (1 W/cm², 2 min) after 6 h of cultivation. The cells were then harvested and disrupted, and the intracellular GSH content was quantified as described in the “GSH and H2O2 consumption” section.
Detection of macrophage polarization
RAW264.7 cells were seeded and cultured in a 12-well plate (1 × 105 cells/well). Then, the cells were polarized into the M2 phenotype using IL-4 (20 ng/mL) and to the M1 phenotype using LPS (100 ng/mL) for 24 h [22]. The cells are stained with APC-CD80 and PE-CD206, and flow cytometry analysis is performed to confirm the successful induction of M1 and M2 phenotypes. CuTT, P/CuTT, and LP/CuTT (50 µg/mL) were added to M2 macrophages, followed by a 680 nm laser (1 W/cm2, 2 min) treatment after 6 h. After an additional 6 h of cultivation, the polarization of M1 and M2 macrophages was detected using the aforementioned methods.
Detection of mitochondrial membrane potential (MMP) and cell apoptosis
MMP was measured using Rhodamine 123 dye. B16-F10 cells were inoculated in 12-well plates with cover slips. Cells were treated with CuTT, P/CuTT, and LP/CuTT (50 µg/mL), followed by a 680 nm laser (1 W/cm², 2 min) treatment after 6 h. The cells were then stained with Hoechst 33342 (10 µM) and Rhodamine 123 (10 µM) dyes, respectively, and subsequently imaged using a CLSM. Cell apoptosis was analyzed using flow cytometry with Annexin V-FITC/PI staining kit.
Scratch assay
Cell migration was evaluated using a scratch assay. B16-F10 cells were seeded into 6-well culture plates at a density of 2 × 10⁵ cells per well and cultured until reaching desired confluency. A straight wound was created in the cell monolayer using a sterile 1000 µL pipette tip. After removing the floating cells by washing thrice with PBS, the cells were incubated in medium containing either CuTT, P/CuTT or LP/CuTT. Wound closure was monitored by optical microscopy, and the wound area was quantified using ImageJ software by normalization to the initial gap area. The migration rate was calculated using the following formula:
$${\rm{Migration\,rate}}\left( {\rm{\% }} \right) = {{{{\rm{A}}_0}-{{\rm{A}}_1}} \over {{{\rm{A}}_0}}} \times 100{\rm{\% }}$$
where A0 represents the initial wound area, and A1 represents the remaining area after 24 h.
Western blotting
B16-F10 cells were seeded into 6-well plates (1 × 106 cells/well). After cultivation for 12 h, CuTT, P/CuTT, and LP/CuTT (50 µg/mL) were added. Following another 12 h of cultivation, cells were collected and protein extraction was performed using a whole protein extraction kit. Protein concentrations were quantified using the BCA method. After boiled in loading buffer, protein samples were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) on pre-cast Bis-Tris 12% gels. Proteins were transferred onto 0.22 μm polyvinylidene fluoride (PVDF) membrane and blocked with 5% BSA in Tris buffered saline tween (TBST) for 1.5 h at room temperature. The membranes were then incubated at 4 °C overnight with primary antibodies including DLAT (1:1000), FDX1 (1:1000), or β-actin (1:2000). After washing three times with TBST, membranes were incubated with HRP-conjugated secondary antibodies (1:10000) for 1 h at room temperature. The protein bands were finally visualized with an ECL substrate kit.
Immunofluorescence assay of CRT and HMGB1
B16-F10 cells were inoculated into a 24-well plate containing slides and treated with CuTT, P/CuTT, and LP/CuTT (50 µg/mL). After 12 h of cultivation, the treated cells were fixed with 4% formaldehyde for 10 min and then sealed with a blocking solution for 30 min. Then, the cells were incubated with CRT antibody for 2 h, followed by incubation with Alexa Fluor® 488 at 37 °C for another 30 min [23]. The same procedure was applied for HMGB1 antibody. Finally, cells were stained with Hoechst 33342 before observation using CLSM.
Bone marrow-derived dendritic cell (BMDC) maturation assay
BMDCs were derived from bone marrow cells isolated from the femurs of female C57 mice (6–8 weeks) [24]. After filtering out tissue fragments and lysing red blood cells, BMDCs were induced using IL-4 (10 ng/mL) and GM-CSF (20 ng/mL). B16-F10 cells were seeded and cultured in 12-well plates, and treated with CuTT, P/CuTT, and LP/CuTT (50 µg/mL), followed by 680 nm laser irradiation. After 12 h of cultivation, the cell culture supernatant was collected and mixed with fresh RPMI-1640 medium containing 10% FBS at a 1:1 ratio [25]. This mixture was then added to pre-seeded BMDCs in 6-well plates and cultured for an additional 24 h. Subsequently, the cells were stained with APC-CD80 and FITC-CD86 antibodies. The maturation of BMDCs was detected using flow cytometry.
Hemolysis assay
Red blood cells were collected from ICR mice and placed in centrifuge tubes containing sodium citrate solution. After centrifugation at 1500 rpm for 10 min, the collected red blood cells were washed 3 times with saline, and then diluted to a 2% suspension with normal saline. A 0.5 mL aliquot of this suspension was mixed with deionized water (ddH2O), saline, CuTT, P/CuTT, and LP/CuTT (0.5 mL) at final concentrations of 200 µg/mL. Red blood cell suspensions treated with saline and ddH2O were served as negative and positive controls, respectively. After 1 h of incubation at room temperature, the mixture was centrifuged at 10,000 rpm, and the absorbance of the supernatant was measured at 540 nm using a microplate reader. The hemolysis ratio was calculated with the following Formula [26]:
$${\rm{Hemolysis}}\left( {\rm{\% }} \right) = {{{{\rm{A}}_{{\rm{sample}}}} - {{\rm{A}}_{{\rm{negative}}}}} \over {{{\rm{A}}_{{\rm{positive}}}} - {{\rm{A}}_{{\rm{negative}}}}}} \times 100{\rm{\% }}$$
Animals
Female ICR mice (6–8 weeks) were purchased from the Wushi Experimental Animals Center (Fuzhou, China). All related animal experimental procedures were carried out according to the protocols approved by the Institutional Animal Care and Use Committee of Fuzhou University.
Establishment of tumor model
The B16-F10 melanoma model was established by subcutaneous injection of B16-F10 cells (5 × 106 cells, 100 µL) into the right flank of the female ICR mice. The mice were monitored daily for tumor growth and general health status.
In vivo imaging of biodistribution
For in vivo fluorescence imaging studies, nanoparticles were labeled with ICG. Briefly, CuTT, P/CuTT and LP/CuTT were dispersed in an aqueous solution containing ICG (500 µg/mL) and subjected to dialysis for 6 h. The hydrodynamic diameter of ICG-labeled nanoparticles was characterized using DLS.
ICG alone and ICG-labeled nanoparticles (ICG-P/CuTT and ICG-LP/CuTT) were administered to ICR mice via tail vein injection at a dose of 1.5 mg/kg in 100 µL volume. Fluorescence imaging was performed at predetermined time points (1, 2, 4, 6, 8, 10, 12, 24, and 48 h post-injection) using a PerkinElmer Caliper IVIS Lumina XR III imaging system (Waltham, Massachusetts, USA).
In vivo antitumor efficacy
Thirty mice with subcutaneous melanoma cancer model were randomly divided into five groups (n = 6), including a saline control group, P/CuTT, P/CuTT with laser irradiation (P/CuTT + L), LP/CuTT, LP/CuTT with laser irradiation (LP/CuTT + L). Once the tumors reached approximately 100 mm3, the B16-F10 tumor-bearing mice were intravenously injected with saline, P/CuTT (1.5 mg/kg), or LP/CuTT (1.5 mg/kg), every 2 days for a total of three treatments. The tumor volume and body weight of the mice were measured every 2 days for 17 days. The laser treatment groups received laser irradiation (680 nm, 1.0 W/cm2, with an effective exposure of 3 min) 10 h post-injection. The tumor volume was measured using a vernier caliper and calculated with the formula V = (a × b2)/2, where a is the long axis and b is the short axis. The mice were sacrificed on the 17th day.
Detection of ·OH and apoptosis (TUNEL) in tumor
B16-F10 tumor-bearing mice were intravenously injected with saline, P/CuTT, or LP/CuTT (1.5 mg/kg). After 10 h, a laser (680 nm, 1.0 W/cm2, with an effective exposure of 3 min) was applied. The laser was removed for 30 s after each 1 min exposure to prevent skin burns on the mice. Subsequently, tumor tissues were sliced using a frozen sectioning machine. HPF (10 µM) and Terminal deoxynucleotidyl transferase dUTP Nick-End Labeling (TUNEL) apoptosis detection kit were used to detect ·OH and tumor cell apoptosis respectively. Finally, Hoechst 33342 was used for staining before observation with a CLSM.
Histopathology analysis
Tumor tissues and major organs (heart, liver, spleen, lungs, and kidneys) were collected after 17 days of treatment, fixed in 4% paraformaldehyde solution, embedded in paraffin, sectioned, and stained with H&E. The stained sections were then examined under a fluorescence microscope (Zeiss, Germany).
Analysis of immune cells and cytokine production
Tumor tissues and spleens were digested to create single-cell suspensions after 17 days of treatment. The cell suspensions were stained with FITC-CD3/APC-CD4 for CD4+ T cells, FITC-CD3/PerCP-CD8a for CD8+ T cells, and FITC-CD11b/APC-CD80 for M1 macrophages for 30 min at room temperature. Following staining, the cell suspensions were washed three times with PBS and analyzed using flow cytometry. Thirty thousand events were recorded for each sample, and the data were analyzed using FlowJo software. The levels of IL-10, IL-1β, and TNF-α in serum were measured according to the instructions provided with the assay kits.
All statistical analyses were performed using GraphPad Prism 10 (GraphPad Software). Experimental data are expressed as mean ± standard deviation (SD) for at least three independent biological replicates. For comparisons between two groups, a two-sided Student’s t-test was applied. For multiple group comparisons, either one-way or two-way analysis of variance (ANOVA) was conducted. Statistical significance was defined as p < 0.05. Significance levels are indicated as follows: *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001, ns (not significant).
CuTT, P/CuTT, and LP/CuTT were synthesized via a conventional self-assembly method (Scheme 1). Previous studies have reported that Te [19] or TCPP [17] can coordinate with Cu2+. We therefore investigated whether Te and Cu2+ (Te + Cu), TCPP and Cu2+ (TCPP + Cu), or Te and TCPP and Cu2+ (CuTT) could self-assemble into nanostructures under mild aqueous conditions. UV–vis spectroscopy revealed that Te + Cu and CuTT showed a red shift in the characteristic absorption peak of tetracycline from 356 nm to 367 nm (Figure S1A), indicating the coordination of Cu2+ with Te. In contrast, TCPP + Cu showed no significant absorption peak shift (Figure S1B). After 1 h incubation at room temperature, Te + Cu and CuTT remained stable, while TCPP + Cu showed substantial precipitation (Figure S1C). DLS measurements demonstrated that Te + Cu exhibited bimodal distribution with peaks at 70 nm and 500 nm, indicating the formation of nanostructures with non-uniform sizes. In contrast, CuTT displayed a single, uniform peak at 150 nm (Figure S1D), while TCPP + Cu displayed peaks exceeding 1000 nm, suggesting no nanostructure formation. These results indicated that Cu2+ assembles with TCPP in an uncontrolled manner in an aqueous environment. In current studies involving Cu2+ and TCPP, nanoparticle synthesis typically occurs in organic reagents, where Cu2+ is loaded into the center of TCPP [27]. In mild environments, only weak interactions between carboxyl groups and the metal were observed (Figure S1). However, when Cu2+, Te, and TCPP were present together, nanostructures with uniform particle size were successfully obtained. The presence of Cu2+ facilitated interactions with both Te and TCPP, thus stabilizing the nanosystem.
To enhance the stability of CuTT, we introduced PVP as a stabilizer and LA as a functional responsive coating. PVP was first added to CuTT to form P/CuTT, followed by LA addition after dialysis to obtain LP/CuTT. DLS analysis showed a homogeneous distribution with particle sizes of 140 nm (CuTT), 135 nm (P/CuTT), and 160 nm (LP/CuTT) in aqueous solution morphology (Fig. 2A). In PBS, particle sizes increased but remained below 300 nm (Figure S2A). However, on the third day, the particle size of CuTT exceeded 300 nm (Fig. 2B) with visible precipitation (Figure S2B). These results demonstrated that the use of PVP or LA significantly improves the stability of nanoparticles in PBS. Next, we used different competing reagents to preliminarily explore the key forces involved in the self-assembly of nanoparticles. As shown in Figure S3, DMSO and EDTA had a significant impact on the stability of CuTT, indicating that π-π stacking and metal ions play a dominant role in the assembly. P/CuTT and LP/CuTT were primarily influenced by DMSO, EDTA, and Tween-20. Therefore, the introduction of PVP and LA suggests that hydrophobic interactions also contribute to the assembly of nanoparticles, thereby providing enhanced stability (Fig. 1B).
Characterization of LP/CuTT. (A) Representative particle size distribution of CuTT, P/CuTT, and LP/CuTT in water. (B) Variation in particle size over three days in a saline environment. (C) TEM morphological characterization of CuTT, P/CuTT, and LP/CuTT. (D) UV spectroscopy results of CuTT, P/CuTT, and LP/CuTT. (E) XPS elemental analysis of CuTT, P/CuTT, and LP/CuTT
Subsequently, the nanoparticles were synthesized in an aqueous environment, and their morphological characteristics were systematically characterized using TEM. As shown in Fig. 2C, TEM revealed uniform particle morphology. The particle sizes observed by TEM were slightly smaller than the DLS-measured sizes. UV–vis spectroscopy demonstrated linear relationships between absorbance and concentration for Te and TCPP quantification (Figure S4). CuTT, P/CuTT and LP/CuTT all exhibited the characteristic peak of Te which is red-shifted from 356 nm to 367 nm due to Cu2+ binding (Fig. 2E), and the typical peaks of TCPP (Fig. 2E inset). XPS analysis confirmed the presence of C, N, O, and Cu elements in all formulations (Fig. 2F), with additional S signals in LP/CuTT due to LA incorporation (Figure S5). In the Cu 2p spectra, XPS peaks with binding energies at 931.8 (Cu 2p3/2) and 953.8 eV (Cu 2p1/2) were assigned to Cu+. In contrast, Cu2+ species exhibited peaks at 935 eV (Cu 2p3/2) and 955.1 eV (Cu2p1/2), along with two characteristic satellite peaks at 942.0 eV and 944.5 eV, respectively [28]. Estimating the relative proportions of Cu in different valence states based on peak area ratios. The Cu2+/Cu+ ratio evolved from pure Cu2+ (CuTT) to 1.26:1 (P/CuTT) and finally 1:1.6 (LP/CuTT) (Figure S5), indicating that PVP stabilized the nanoparticles while LA reduced Cu2+ to Cu+ due to the reducing properties of its disulfide bonds [29]. Both Cu2+ and Cu+ are important for the Fenton-like reaction in cells. However, Cu+ demonstrates superior Fenton-like reaction efficiency compared to Cu2+ and can exert a more potent CDT [30]. Therefore, LP/CuTT is expected to achieve enhanced anti-tumor effects.
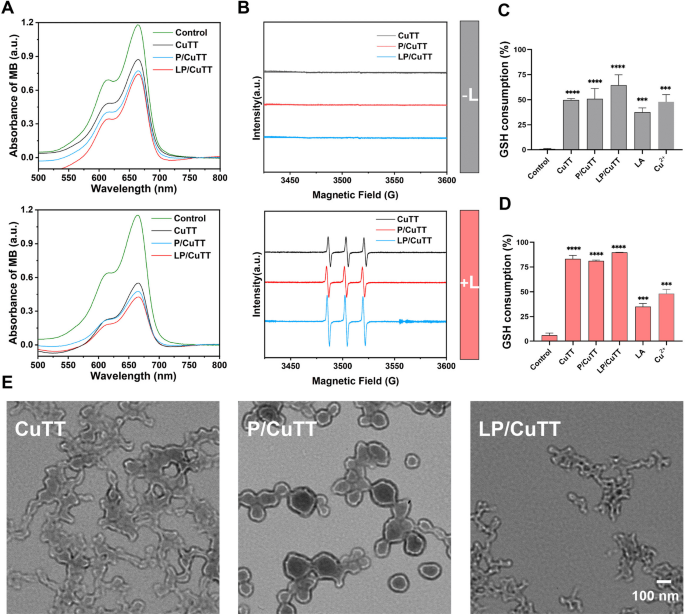
MB can be bleached by ·OH, making it an effective indicator for monitoring the generation of ·OH. Due to the elevated concentration of H2O2 in TME, we measured the material’s ability to generate ·OH in this context. In the presence of H2O2, Cu2+ can degrade MB both with and without light irradiation (Figure S6), indicating that Cu2+ can catalyze the generation of ·OH from H2O2. In the absence of light, CuTT, P/CuTT, and LP/CuTT all induced MB bleaching (Fig. 2A), which could be attributed to the presence of copper ions [31]. When a 680 nm laser was applied, increased MB bleaching was observed, indicating elevated ·OH generation. The present study observed the highest ·OH yield in LP/CuTT, followed by P/CuTT, while CuTT exhibited the lowest yield. According to the reaction mechanisms (Eqs. 1–3), Cu2+ must first be reduced to Cu+ before reacting with H2O2 to generate ·OH. The superior performance of LP/CuTT can be attributed to its highest concentration of Cu+, which facilitated the most efficient ·OH generation. As shown in Figure S7, the LP/CuTT group consumed more H2O2 than both the CuTT and P/CuTT groups. However, in the presence of Cu2+ alone, no significant H2O2 degradation was observed within 10 min. This can be attributed to the fact that Cu2+ requires an additional reaction step compared to Cu for ·OH production. LA also caused the depletion of H2O2, probably due to its reducing properties. Additionally, 1O2 production was monitored using TEMP as a ROS scavenger. ESR spectroscopy revealed a distinct characteristic peak for 1O2 following 680 nm laser irradiation (Fig. 2B), with LP/CuTT demonstrating enhanced 1O2 production. Notably, no characteristic 1O2 peak was detected in the absence of laser stimulation, confirming that 1O2 formation is specifically induced by the photosensitizer TCPP.
ROS production and GSH responsiveness. (A) Degradation of MB in each treatment group under H2O2 conditions with or without 680 nm laser treatment. (B) EPR measurement of 1O2. (C-D) GSH consumption with or without laser treatment. (E) TEM morphology after exposure to GSH conditions. L: laser irradiation. ***p < 0.001, ****p < 0.0001, compared with control
Subsequent studies investigated the ability of LP/CuTT to deplete GSH. The results showed that in the absence of light, GSH was consumed in both P/CuTT and LP/CuTT, with LP/CuTT representing the highest consumption rate (Fig. 2C). This consumption occurred as GSH was oxidized to GSSH by Cu2+ (Eq. 1), consistent with findings that Cu2+depletes GSH (Fig. 2C-D). Upon laser irradiation, GSH levels decreased further, suggesting that ROS produced during this process contributed to increased GSH consumption. Additionally, GSH reduction was observed in the presence of LA, which could clarify why the LP/CuTT group exhibited higher GSH consumption than the CuTT and P/CuTT groups. GSH responsiveness plays a crucial role in achieving selectivity in cancer cells, as cancer cells typically overexpress GSH. To evaluate the redox-responsive behavior of the nanoparticles, we examined the morphological changes of nanoparticles in GSH-containing solutions. TEM analysis revealed distinct morphological changes among the nanoparticle formulations following GSH exposure. Unmodified CuTT nanoparticles exhibited significant aggregates exposed to GSH, while PVP-stabilized P/CuTT nanoparticles maintained colloidal stability with only moderate size reduction. In contrast, LP/CuTT nanoparticles displayed a distinct structural collapse, which was significantly different from that of CuTT nanoparticles (Fig. 2E). The results above demonstrate that PVP incorporation significantly improves nanoparticle stability. Importantly, LA loading maintains this stability under physiological conditions (Fig. 2B) while simultaneously conferring GSH-responsive properties to LP/CuTT. This characteristic holds significant importance for the design of TME-responsive nanoparticles.
The reaction processes involving copper ions in this system are summarized as follows [32]:
$$C{u^2}^ + + GSH \to C{u^ + } + GSSH $$
$$C{u^2}^ + + {H_2}{O_2} \to C{u^ + } + \cdot {O_2}H + {H^ + } $$
$$C{u^ + } + {H_2}{O_2} \to \cdot OH + O{H^ - } + C{u^2}^ + $$
Mouse melanoma B16-F10 cells and embryonic mouse fibroblast NIH/3T3 cells were used for the in vitro toxicity analysis. To determine the optimal light exposure time post-administration, the uptake of nanoparticles in B16-F10 cells was measured at 2, 4, and 6 h post-treatment. Flow cytometry results showed that the uptake of B16-F10 cells tended to saturate after 4 h of material administration, with a slight increase at 6 h (Fig. 3A, S8A). Therefore, we executed laser stimulation 4 h after nanoparticle administration. The LP/CuTT group exhibited significantly higher cellular uptake compared to other groups (Fig. 3B). To investigate the mechanisms underlying this enhanced uptake, we used PI, a standard marker for dead or membrane-permeable cells. B16-F10 cells were treated with either LA or PBS, and the uptake of PI dye was monitored at various time points. Results showed that LA pre-treatment significantly enhanced the uptake of PI by B16-F10 cells (Fig. 3C, S8B). Notably, no significant cytotoxicity was observed even at concentrations up to 400 µg/mL (Fig. 4D). All this evidence suggests that LA modification enhances cell membrane permeability without compromising cell viability, thereby facilitating increased nanoparticle uptake.
Cellular uptake and cytotoxicity analysis of LP/CuTT. (A) Comparison of time required to achieve maximum cellular uptake across different treatment groups. (B) Time-dependent intracellular uptake of CuTT, P/CuTT, and LP/CuTT in B16-F10 cells. (C) Time-course analysis of PI uptake in B16-F10 cells after LA treatment. (D) MTT cytotoxicity assay results for NIH/3T3 cells exposed to various concentrations of LA. (E) MTT cytotoxicity assay on B16-F10 cells treated with CuTT, P/CuTT, and LP/CuTT with and without exposure to a 680 nm laser. (F) Relative viability of NIH/3T3/B16-F10 cells under different treatment conditions
The cytotoxicity of CuTT, P/CuTT, and LP/CuTT against B16-F10 and NIH/3T3 cells was initially assessed using the MTT assay. The results (Fig. 4E) showed that at a concentration of 50 µg/mL, the toxicity observed in NIH/3T3 cells under dark conditions maintained a viability of approximately 75% (Figure S9), indicating a weak cytotoxicity. In contrast, cytotoxicity towards B16-F10 cells remained high at around 60%. These findings align with previous studies reporting differential lethal effects of Te between normal and cancer cells [33]. Comparative analysis of in vitro cell viability between CuTT, P/CuTT and LP/CuTT at equivalent concentrations under dark conditions revealed significantly higher cytotoxicity against B16-F10 cells compared to NIH/3T3 cells at 50 µg/mL (Fig. 4F). The difference between two strains of cells may be due to the presence of Te, which exhibits selective toxicity toward tumor cells over normal cells. Further investigation of Te concentration-dependent effects on both cell lines (Figure S10A) revealed that B16-F10 cells exhibited enhanced sensitivity to Te exposure compared to NIH/3T3 cells. Additional cytotoxicity studies of individual components showed no significant difference in the toxicity of Cu2+ or TCPP on B16-F10 cells and NIH/3T3 cells (Figure S10B, S11). These results further confirm that the differential killing properties of CuTT, P/CuTT and LP/CuTT against NIH/3T3 and B16-F10 cells are primarily due to the presence of Te.
Upon 680 nm laser irradiation, the cell viability of B16-F10 decreased substantially to 45.63%, 36.53%, and 23.80% for CuTT, P/CuTT, and LP/CuTT, respectively (Fig. 4E), confirming their photo-facilitated therapeutic effects. To evaluate the photo-enhanced chemodynamic therapeutic effects of CuTT at the cellular level, ROS production was evaluated using DCFH-DA dye. CLSM demonstrated significant ROS generation within cells following different nanoparticle treatments (Fig. 4A). Quantitative analysis (Fig. 4B) showed increased ROS production after 680 nm laser irradiation. Notably, LP/CuTT showed stronger fluorescence signals, possibly due to enhanced cellular uptake. As a critical type of ROS, ·OH has been the focus of numerous studies aiming to enhance their yields for improved tumor-killing effects [35]. Therefore, we assessed the capability of CuTT, P/CuTT, and LP/CuTT to trigger ·OH production in the absence or presence of laser stimulation in tumor cells using HPF dye. Results indicated that ·OH generation occurred independently of laser stimulation (Fig. 4C). Further investigation of the capacity of the individual and combined components to induce ·OH revealed that the interaction between Te, TCPP, and Cu2+ synergistically enhanced ·OH production (Figure S12), which is primarily through the inherent Fenton-like reaction of Cu2+. In addition, TCPP independently induced ·OH generation, and additional illumination further amplified the generation, which is likely due to the unique structure of porphyrins [36]. However, Te alone did not contribute to the increased production of ·OH (Figure S12). The synergistic integration of TCPP and Te resulted in a marked enhancement of Cu2+-mediated ·OH generation, thereby amplifying the intracellular CDT response. This effect was further potentiated by 680 nm laser irradiation. Compared to the CuTT group, both P/CuTT and LP/CuTT exhibited enhanced ·OH generation upon 680 nm laser irradiation, with LP/CuTT demonstrating the highest yield. The ·OH generation efficiency of LP/CuTT reached 57.2% under 680 nm laser irradiation, representing about 1.5-fold enhancement compared to the CuTT control group.
Assessment of intracellular ROS production, GSH levels and cell migration. (A) Confocal images and (B) quantitative analysis of fluorescence intensity using Image J software to determine total intracellular ROS production in B16-F10 cells treated with CuTT, P/CuTT, and LP/CuTT with and without exposure to a 680 nm laser. (C) Hydroxyl radical assay results for B16-F10 cells following different treatments. (D) Quantification of GSH depletion in B16-F10 cells after different treatments. (E) Wound healing assay images and (F) quantitative analysis of cell migration rates using Image J software after different treatments. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001, compared with control group without laser irradiation. #p < 0.05, ns indicates no significant difference
Excessive ROS production typically leads to the depletion of intracellular reducing substances, such as GSH. Analysis of GSH levels in B16-F10 cells after different treatments showed a significant depletion (Fig. 5D), indicating disruption of cellular redox homeostasis. Melanoma is characterized by its strong propensity for metastasis, particularly to the brain and lungs. We examined the capacity of nanoparticles to impede the migration of B16-F10 cells. The cell scratch assay demonstrated that CuTT, P/CuTT, and LP/CuTT significantly inhibited B16-F10 cell migration (Fig. 5E, F). Among these, P/CuTT and LP/CuTT demonstrated superior inhibitory efficacy. It has been reported that certain tetracycline antibiotics can inhibit tumor cell migration [36]. Similarly, TCPP and Te also exhibited excellent anti-metastatic effects (Figure S13). However, precipitation was observed in the TCPP treatment group during cell culture.
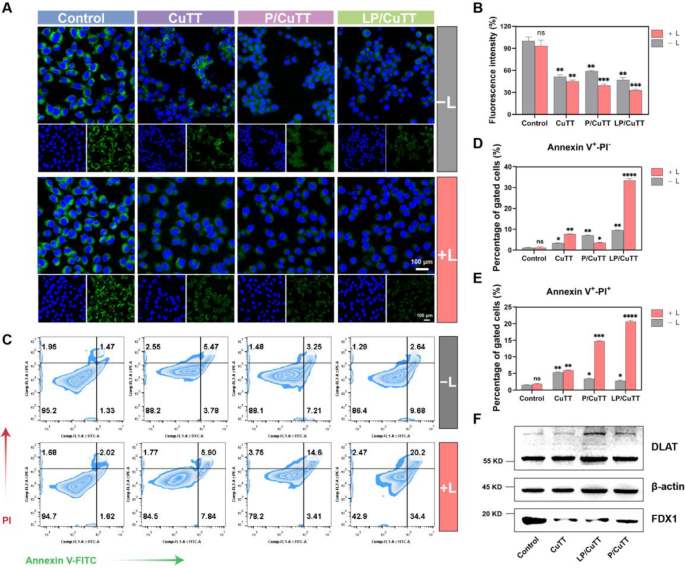
Given that high ROS levels can damage mitochondria, we assessed the MMP to evaluate mitochondrial function. After various treatments, a decrease in MMP levels was observed (Fig. 5A). Quantitative fluorescence analysis revealed comparable MMP reduction across CuTT, P/CuTT, and LP/CuTT treatment groups under dark conditions, with no statistically significant differences between groups (Fig. 5B). Following 680 nm laser irradiation, P/CuTT and LP/CuTT exhibited a more pronounced reduction in MMP. Mitochondria play a crucial role in energy production, calcium homeostasis, and iron metabolism, and are integral to intrinsic apoptosis via cytochrome release [37]. Flow cytometry results showed that LP/CuTT effectively induced apoptosis (Fig. 5C), and the additional laser irradiation can lead to a significant increase in apoptosis. This is because the additional laser irradiation led to increased ROS production, causing MMP loss and triggering apoptosis. Flow cytometric analysis revealed comparable increases in apoptosis rates between the CuTT and P/CuTT groups under dark conditions (9.45% vs. 10.73%), while LP/CuTT exhibited a marginal upward trend (12.35%). Photoirradiation induced distinct apoptotic modulation: P/CuTT preferentially enhanced late-stage apoptosis in B16-F10 cells (18.62% vs. CuTT’s 13.42%), whereas LP/CuTT demonstrated concurrent amplification of both early (33.51%) and late apoptotic populations (20.64%), representing about 4.4- and 3.5-fold increases, respectively, compared to the CuTT group (Fig. 5D, E). The differences in apoptotic outcomes between the P/CuTT and LP/CuTT groups could be attributed to the presence of LA, which promotes cellular uptake, leading to greater ROS generation, mitochondrial dysfunction, and increased apoptosis.
Induction of MMP collapse, apoptosis and cuproptosis by LP/CuTT. (A) Representative confocal microscopy images and (B) quantitative analysis of Rhodamine 123 fluorescence intensity using ImageJ software to assess MMP in B16-F10 cells treated with CuTT, P/CuTT, and LP/CuTT with and without exposure to a 680 nm laser. (C) Flow cytometric analysis of cell apoptosis following different treatments, and the corresponding statistical analyses of (D) early apoptosis cells and (E) late apoptosis/necrotic cells. (F) Western blot analysis of DLAT and FDX1 protein contents in B16-F10 cells after different treatments. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001, compared with Control group without laser irradiation
In the tricarboxylic acid cycle, the binding of copper ions to lipoylated proteins induces the oligomerization of lipoylated proteins, a key step in the cuproptosis pathway. FDX1 acts as a reductase that reduces Cu2+ to Cu+ and regulates the lipoylation of DLAT [38]. Analysis of FDX1 and DLAT protein expression in treated B16-F10 cells showed decreased FDX1 levels compared to controls, while DLAT oligomerization was observed (Fig. 6F), which can be hypothesized that the nanoparticles induced the process of cuproptosis.
The clinical treatment of melanoma still faces numerous challenges, including poor prognosis, as neither monotherapy nor combination therapy has demonstrated optimal efficacy [39]. Although immunotherapies such as anti-programmed death-ligand 1 (PD-L1) and anti-cytotoxic T-lymphocyte-associated protein 4 (CTLA4) have achieved many unexpected breakthroughs in cancer treatment, they still present significant limitations [40]. In this section, we tested the ability of CuTT, P/CuTT, and LP/CuTT to induce immune responses in vitro (Fig. 6A). The objective was to ascertain the capacity of the nanoassemblies to promote DC maturation and to establish an M2-type RAW 264.7 cell model for assessing the induction of M1-type pro-inflammatory macrophages. Recent studies have shown that cuproptosis can cause ICD, which in turn triggers immunity [6]. Key proteins involved in this process include HMGB1 and CRT. Immunofluorescence assay (Fig. 6B) demonstrated that HMGB1, which is normally located in the cell nucleus, was released into the outside of the cell, accompanied by a significant increase in the level of CRT on the cell membrane. In the absence of light exposure, B16-F10 cells treated with CuTT, P/CuTT, and LP/CuTT exhibited a substantial release of HMGB1, concomitant with a decline in green fluorescence intensity. Further analysis revealed that 680 nm laser treatment led to a pronounced decrease in fluorescence intensity, suggesting a potential loss of HMGB1 from the cells. However, no statistically significant differences were observed among the treatment groups. The release of CRT resulted in the induction of ICD. Detection of CRT exposure revealed that the LP/CuTT group exhibited the highest levels, followed by the P/CuTT group, and finally the CuTT group. This trend was consistent, with a greater amount of CRT exposure observed under light conditions. CRT acts as both a “find me” and “eat me” signal, facilitating interactions with antigen-presenting cells to accurately localize dead cells within tissues and recruit immune cells into the TME. By binding to specific receptors on DCs, HMGB1 stimulates efficient processing and cross-presentation of tumor antigens in dead cells, thereby mediating potent pro-inflammatory effects [41].
In vitro immunomodulatory effects of LP/CuTT. (A) Schematic representation of dendritic cell activation in vitro and the mechanism of dendritic cell activation by induction of macrophage polarization and ICD effect in B16-F10 cells. (B) Immunofluorescence assay of CRT and HMGB1 in B16-F10 cells induced by CuTT, P/CuTT, and LP/CuTT with or without 680 nm laser treatment. (C) Determination of the ability to induce macrophage polarization from M2 to M1 in different treatment groups. (D) Evaluation of dendritic cell maturation after different treatments
TAMs are one of the major immunomodulatory cell types within tumors. M2 anti-inflammatory TAMs contribute to the immunosuppression of the TME, including the inhibition of DCs maturation and suppression of cytotoxic T lymphocyte responses. To counteract the immunosuppressed TME, it is essential to repolarize TAMs from the M2 to the M1 pro-inflammatory phenotype. We evaluated the ability of the nanoassemblies to induce M1 polarization in RAW264.7 macrophages using IL-4-induced RAW264.7 cells as the M2 type and LPS-induced cells as the M1 type. After the model was successfully established (Figure S14A), the macrophage phenotypes were detected after different treatments. The percentages of cells positive for M1 and M2 markers are calculated from the upper left quadrant (Q2) and the lower right quadrant (Q4) of the scatter diagrams. The Q2/Q4 ratio was used as a score to quantify the degree of macrophage polarization from M2 to M1, with higher scores representing a greater number of macrophages polarized from M2 to M1. CuTT, P/CuTT, and LP/CuTT received scores of 1.99, 1.44, and 1.64, respectively (Fig. 7C and S15), which increased to 2.90, 2.38, and 2.84 after laser administration. These results suggest that LP/CuTT can effectively promote the polarization of macrophages from M2 to M1, which is expected to help regulate the immunosuppressive microenvironment within the tumor. A previous study reported that a tetracycline antibiotic can promote M1 polarization of macrophages [18], and we also determined that Te can promote the polarization of macrophages from M2 to M1 (Figure S14B).
DCs are one of the most important antigen-presenting cells, which play an important role in both the innate and adaptive immune system. Mature DCs can express co-stimulatory molecules, secrete cytokines, and migrate to lymph nodes to initiate the priming of naive T cells [42]. The exposure of CRT serves as a signal that triggers the phagocytosis of dead cellular debris by DCs, and the release of HMGB1 can facilitate the association between tumor cells with DCs, promoting further cell death. Based on the results of LP/CuTT-induced ICD in B16-F10 cells from the immunofluorescence experiments (Fig. 7B), we therefore used BMDCs to investigate whether LP/CuTT-induced ICD could promote the maturation of BMDCs. We first incubated the nano assemblies with B16-F10 cells, then collected the supernatant, mixed it with fresh medium in a 1:1 ratio, and used this mixture to culture immature BMDCs. As expected, CuTT, P/CuTT and LP/CuTT significantly increased BMDCs maturation compared to the control group (Fig. 6D). Among these, LP/CuTT exhibited the most pronounced effect on BMDCs maturation, achieving maturation rates of 50.3% with laser treatment and 31.2% without it. The enhancement observed with laser irradiation is likely due to laser treatment promoting the release of HMGB1 and CRT from B16-F10 cells, thus further enhancing BMDCs maturation. In conclusion, the results suggest that P/CuTT and LP/CuTT can stimulate robust immune responses.
Hemolysis tests were conducted before the animal experiments. The results showed that P/CuTT and LP/CuTT exhibited hemolysis rates below 5%, while CuTT showed a hemolysis rate of 25% (Figure S16), making it unsuitable for tail vein injection in mice. The difference in hemolysis rate may be attributed to the shielding of surface charges on CuTT by PVP and LA modifications (Figure S2A). Consequently, we proceeded with animal experiments to evaluate the antitumor efficacy of P/CuTT and LP/CuTT. The diagram of the animal experimental program is illustrated in Fig. 7A. Based on previous research [18] and comprehensive biosafety considerations, the animal dosing concentration was selected as 1.5 mg/kg.
In vivo antitumor efficacy of LP/CuTT in B16-F10 tumor-bearing mice. (A) Schematic illustration of the treatment schedule. (B) In vivo fluorescence imaging showing the biodistribution of free ICG, ICG-P/CuTT, and ICG-LP/CuTT in tumor-bearing mice at different time points post-injection. (C) Quantitative analysis of fluorescence intensity at tumor sites at various time points for each treatment group. (D) Tumor growth curves for different treatment groups. (E) Photographs of excised tumors from different treatment groups. Groups are labeled as follows: (1) Control, (2) P/CuTT, (3) P/CuTT with laser irradiation (P/CuTT + L), (4) LP/CuTT, (5) LP/CuTT + L. Red circle, tumor-free mouse. (F) HPF and TUNEL staining of tumor sections from different groups with Hoechst 33342 staining (blue) showing cell nuclei
We established a B16-F10 melanoma tumor model on the right flank of ICR mice. Free ICG, ICG-labeled P/CuTT, and ICG-labeled LP/CuTT were administered to tumor-bearing ICR mice through the tail vein. DLS analysis confirmed that ICG labeling had minimal impact on nanoparticle size (Figure S17). LP/CuTT demonstrated significant tumor accumulation at 6 h post-injection (Fig. 8B), which peaked at 10 h. This enhanced targeting capability can be attributed to the favorable GSH response properties of LA (Fig. 2E). As shown in Figure S3, LA contributes hydrophobic interactions during nanoparticle assembly. When exposed to elevated GSH levels in the TME, cleavage of disulfide bonds in LA may lead to structural destabilization of the nanoparticles, potentially facilitating enhanced tumor accumulation. In contrast, free ICG exhibited no tumor targeting effect and had a short in vivo retention time. Additionally, since PVP lacks any targeting specificity, P/CuTT exhibited modest tumor accumulation at 12 h during circulation, but lacked notable targeting properties (Fig. 8C). The addition of LA endows LP/CuTT with a certain degree of GSH-responsive capability (Fig. 2E) and it provides hydrophobic interaction forces during the assembly process (Figure S3). When the critical disulfide bond in LA is disrupted by GSH, it may lead to structural destabilization of the nanoparticles, which we believe contributes to its enhanced tumor accumulation. Encouraged by the excellent tumor targeting of LP/CuTT, we proceeded to conduct in vivo efficacy experiments. Treatment began via tail vein injection when tumor volumes reached 100 mm3 (Day 1), followed by 680 nm laser irradiation at the tumor site 10 h post-injection. Body weight and tumor volume were monitored every other day throughout the study. No significant changes in body weight were observed in any treatment groups compared to the control group (Figure S18). The saline control group showed rapid tumor growth, necessitating euthanasia of one mouse due to severe distress (Fig. 8D). P/CuTT achieved tumor inhibition rates of 58.57% and 88.99% without and with laser stimulation, respectively. LP/CuTT demonstrated superior efficacy with inhibition rates of 93.41% and 97.92% without and with laser treatment, respectively. Notably, complete tumor regression was observed in three mice from the LP/CuTT plus laser treatment group (Fig. 8E).
Following the third treatment cycle, tumor specimens from each treatment group were harvested and processed into cryosections. Subsequently, tissue sections were subjected to staining with HPF and TUNEL, respectively (Fig. 8F). Confocal microscopy results revealed significantly enhanced ·OH generation at tumor sites in both the P/CuTT and LP/CuTT treatment groups compared to the PBS controls. Notably, LP/CuTT-treated tumors exhibited intense HPF-derived green fluorescence after 680 nm laser irradiation. This occurs because LA, acting as a reducing agent, increases the proportion of Cu+ in the LP/CuTT, as confirmed by XPS analysis in the material characterization section (Figure S5). Compared to Cu2+, Cu+ exhibits higher Fenton-like reaction efficiency, leading to enhanced generation of ·OH at the tumor site. Additionally, laser irradiation further improves the CDT efficiency. TUNEL staining analysis showed no significant apoptotic signals in control tumor tissues. In striking contrast, both the P/CuTT and LP/CuTT treatment groups induced varying degrees of tumor cell apoptosis. HPF investigations demonstrated that even under dark conditions, both nanosystems could continuously generate highly oxidative ·OH, thereby triggering tumor cell death. Notably, upon 680 nm laser irradiation the LP/CuTT group exhibited the most pronounced apoptotic efficacy. The observed apoptosis indices showed strong consistency with the remarkable tumor suppression efficacy (Fig. 8E), confirming the therapeutic superiority of LP/CuTT.
TAMs represent a highly plastic population of immune cells with both pro-tumor and anti-tumor functions. M2-like TAMs often promote immune evasion and tumor progression, whereas M1-like TAMs are associated with anti-tumor responses and elicit immune responses. We determined the levels of M1-like TAMs in both mouse tumor tissue and spleen. As shown in Fig. 8A, the infiltration level of M1-like macrophages was significantly increased in the P/CuTT and LP/CuTT groups compared to the control group, and the level was further enhanced following an additional 680 nm laser treatment (Fig. 8B). Moreover, the LP/CuTT group demonstrated a higher proportion of M1 macrophages than the P/CuTT group, regardless of laser irradiation. Cytotoxic CD8+ T cells are crucial for the elimination of intracellular infections and malignant cells, providing long-term protective immunity [43]. Additionally, CD4+ T cells have emerged as key players in both the initiation and effector phases of the antitumor immune response [44]. Consequently, we assessed the infiltration of T cells in tumor tissue. As illustrated in Fig. 8C-F, although the number of CD8+ T cells and CD4+ T cells at the tumor site tended to increase after P/CuTT treatment, the results were not particularly satisfactory. However, the addition of laser stimulation led to a significant enhancement in the immune response, with the number of CD8+ T cells and CD4+ T cells increasing from 5.45% and 5.75% in the control group to 25.4% and 28.9%, respectively, demonstrating a strongly significant difference. Furthermore, the LP/CuTT group displayed a strong immune response even without laser stimulation, with the number of CD8+ T cells and CD4+ T cells elevated to 16.3% and 24.2%, respectively, and further increased to 29.1% and 33.9% following 680 nm laser stimulation. The results indicate that LP/CuTT possesses a significantly enhanced T-cell infiltration capacity compared to P/CuTT. This enhanced immunostimulatory effect likely stems from its higher ·OH production efficiency, which induces greater tumor cell release of damage-associated molecular patterns (DAMPs), thereby promoting more robust immune cell recruitment within TME. We also measured the levels of M1 macrophages and T cells in the spleen (Fig. 8G-L), and similar results were observed, indicating a robust immunostimulatory capacity. The accumulation of LP/CuTT at the tumor site and the activation of the immune system were found to be beneficial, resulting in an enhanced therapeutic effect in the ICR melanoma tumor model.
In vivo immunomodulatory effects of LP/CuTT. Representative flow cytometric analysis and quantification of tumor-infiltrating M1 TAMs gated on CD11b+ and CD80+ cells (A, B), CD4+ T cells gated on CD3+ and CD4+ cells (C, D), and CD8+ T cells gated on CD3+ and CD8a+ cells (E, F) from the upper right quadrant of the scatter diagrams in tumors after 17 days treatments. G-L) Representative flow cytometric analysis and quantification of M1 TAMs, CD4+ T cells and CD8+ T cells in spleen after different treatments. Serum levels of cytokines M) TNF-𝛼, N) IL-1β, and O) IL-10 in mice after different treatments detected by ELISA. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001, compared to control. #p < 0.05, ns indicates no significant difference
M1 macrophages secrete pro-inflammatory cytokines, including IL-1β, IL-6, and TNF-α, which play crucial roles in pro-inflammatory responses and exhibit potent anti-tumor capacity [45]. We used ELISA to measure the cytokines in the peripheral blood of B16-F10 tumor-bearing mice (Fig. 8M-O). Compared with the control group, we found significant increases in the levels of the pro-inflammatory factors IL-1β and TNF-α, and a dramatic decrease in the immunosuppressive cytokine IL-10. These changes were particularly pronounced in groups treated with P/CuTT and LP/CuTT under laser irradiation. Among all experimental groups, LP/CuTT + L demonstrated the optimal capability in inducing pro-inflammatory factors while suppressing anti-inflammatory factors. These findings suggest a reprogramming of the tumor immunosuppressive microenvironment, leading to the elicitation of a robust immune response. Finally, we performed H&E histopathological analysis on major organs including the heart, liver, spleen, lungs, and kidneys to evaluate biosafety. The results showed no obvious pathological changes in organs (Figure S19), indicating favorable biosafety. The experimental evidence demonstrates that LP/CuTT exhibits strong potential for antitumor applications.
In this study, we developed an orchestrated Cu2+-coordinated tetracycline-porphyrin self-assembly (LP/CuTT) with LA modification to precisely reprogram the immunosuppressive TME and enhance antitumor immunotherapy through photo-enhanced CDT. LP/CuTT demonstrated potent CDT effects through the production of toxic ·OH, leading to apoptosis and substantial cuproptosis in B16-F10 cells. The photo-enhanced CDT mediated by LP/CuTT induced ICD, which promoted dendritic cell maturation and facilitated macrophage polarization toward the M1 phenotype. The LA modification endowed LP/CuTT with redox-responsive properties, leading to enhanced accumulation in the TME. This tumor-specific accumulation resulted in a higher number of M1-type anti-tumor macrophages, along with elevated CD8+ and CD4+ T-cell populations, ultimately contributing to effective tumor suppression.
While the current study demonstrates promising antitumor efficacy, future research should explore long-term TME modulation, potentially by integrating immune checkpoint blockade strategies. Although the composition and preparation of LP/CuTT are relatively complex, their remarkable therapeutic efficacy and multifunctional properties demonstrate great potential for clinical translation. Further optimization of its formulation scalability, along with thorough preclinical safety and biocompatibility assessments, will be vital for clinical translation. Our findings suggest that LP/CuTT holds promise for advancing cancer immunotherapy by repurposing readily available antibiotics and photosensitizers to achieve multi-pronged reprogramming of the immunosuppressive TME. This study provides valuable insights into the development of orchestrated metal-coordinated nanoassemblies for effective nanodynamic therapy that integrate multipronged TME remodeling, multifaceted immune activation, and multimodal tumor suppression.
No datasets were generated or analysed during the current study.
This work was supported by the Natural Science Foundation of Fujian Province, China (2024J01999), the Beijing Science and Technology Innovation Medical Development Foundation of China (KC2023-JX-0288-PM99).
The authors declare no competing interests.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
Sun, X., Wang, X., Li, X. et al. Orchestrated Cu2+-coordinated tetracycline-porphyrin self-assembly remodels tumor microenvironment for photo-enhanced immuno-chemodynamic therapy. J Nanobiotechnol 23, 419 (2025). https://doi.org/10.1186/s12951-025-03486-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12951-025-03486-9