FDA Approves At-Home Cervical Cancer Screening

The Teal Wand, a self-collection device for HPV testing, is set to revolutionize cervical cancer screening. Recently approved by the FDA, it offers a simple at-home test, eliminating the need for speculum exams and doctor’s office visits. This innovation is particularly impactful for women facing barriers to traditional screening methods.
Cervical cancer screening rates have been declining, making early detection crucial. The Teal Wand provides a convenient option that has the potential to reverse this trend and save lives. HPV types 16 and 18 are responsible for approximately 90% of all cervical cancer cases, highlighting the importance of early detection through HPV testing.
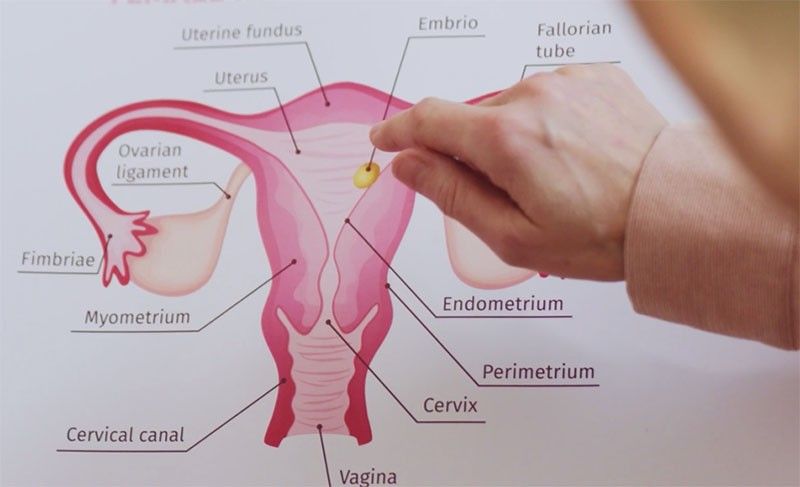
Cervical cancer screening has evolved from primarily relying on Pap tests to incorporating HPV testing. The Pap test examines cells from the cervix for cancerous or precancerous changes, while the HPV test detects high-risk strains of the human papillomavirus. Current guidelines recommend Pap tests starting at age 21, with varying frequencies based on age and test results. Women between 30 and 65 can opt for a Pap test alone, an HPV test alone, or both.
The Teal Wand isn’t the first step towards self-collection. Last year, the FDA approved a self-collected HPV test that still required a healthcare setting. The Teal Wand takes it a step further, allowing women to collect samples at home. Designed for one-handed use, the Teal Wand features a handle, a sponge at the top, and a dial at the bottom to spin the sponge. The user inserts the wand, spins the sponge, detaches it, and sends it to a lab for analysis. Proper technique is crucial for accurate results.
The SELF-CERV study evaluated the Teal Wand’s ease of use and accuracy. Results showed that over 98% of participants collected a valid sample, with 95% of cases matching clinician-collected samples. Additionally, 93% found the device easy to use, and 94% preferred it over a clinician-collected sample.
The Teal Wand benefits women who face barriers to traditional screening, including those lacking transportation, unable to take time off work, or uncomfortable with speculum exams. This is particularly relevant in the United States, where healthcare access varies widely. At-home testing can bridge these gaps and improve health equity.
Future advancements in at-home testing technology are expected, including more user-friendly devices and faster turnaround times. Teal Health plans to offer a telehealth option, as a prescription is required for the test. This integration could become a standard model for healthcare delivery.
However, self-collection is not recommended for pregnant women, those with a history of cervical precancer treatment, HIV-positive individuals, or those with a history of reproductive system cancer. Ensuring equitable access to at-home testing for all women is crucial.
Dr. Anya Sharma emphasized that the Teal Wand represents a potential paradigm shift in cervical cancer prevention by addressing barriers such as lack of transportation, difficulty taking time off work, anxiety surrounding speculum exams, or limited access to healthcare providers. The Teal Wand has the potential to significantly improve screening rates, particularly among underscreened women.
Telehealth integration allows women to consult with a healthcare provider remotely to obtain the necessary prescription, receive guidance on proper usage, and discuss their results, streamlining the process and enhancing accessibility. She also noted that while the Teal Wand is a promising option, it’s essential for women to discuss their individual needs and risk factors with their healthcare provider to determine the most appropriate screening strategy.
Regular screening is a crucial step in preventing cervical cancer and saving lives, and early detection is key.