BMC Cancer volume 25, Article number: 922 (2025) Cite this article
Immunotherapy in the presence of COVID-19 infections raises concerns because of potential overlapping clinical complications and immune system enhancement. Further investigation is warranted to establish its safety and to improve clinical decisions.
We conducted a retrospective cohort study using linked health administrative data from Ontario, Canada to assess 30-day mortality in patients with solid tumors who were treated with immunotherapy within 120 days before testing positive for COVID-19. A stepwise multivariable logistic regression model was used to identify clinical factors associated with 30-day mortality.
Between January 2020 and April 2023, 281 patients tested positive for COVID-19 and were included in our study. The mean age was 68 (Standard Deviation: 10.3), 45% (127/281) were females and 58% (163/281) had lung cancer. 59% of patients (167/281) were treated with single agent immunotherapy, and almost 80% received at least one dose of COVID-19 vaccine. The 30-day mortality was 22% (63/281) and < 5% of patients were admitted to ICU or required ventilation. Factors associated with higher mortality were older age (Odds Ratio (OR) 1.60, 95% confidence interval (CI) 1.07–2.39), prior radiation therapy (OR 2.38, 95%CI 1.08–5.28), lower hemoglobin (< 10 g/dl) (OR 4.08, 95%CI 1.89–8.82) and higher leucocytes count (> 11,000/mm3) (OR 3.63, 95%CI 1.55–8.52).
Immunotherapy does not seem to increase the risk of 30-day mortality in patients with COVID-19 infections compared to published outcomes of patients with cancer and COVID-19. Mortality was associated with certain clinical characteristics that need to be carefully examined when prescribing immunotherapy during future comparable pandemics.
A pandemic related to coronavirus disease (COVID-19) was declared early in 2020 with devastating effects and immense strain on the healthcare system in general and particularly on patients with cancer related to delayed diagnosis and delays in treatment start [1, 2]. Moreover, mortality rates related to COVID-19 seem to be significantly higher in patients with cancer compared to the rest of the population. Several studies including systematic reviews and meta-analyses reported a mortality rate as high as 25 to 30% [3,4,5,6] among patients with cancer. This rate varied depending on age, tumor site and other characteristics.
Patients with cancer might have an augmented immune response to infection secondary to immunomodulatory drugs, such as programmed cell death 1 or programmed cell death ligand 1 inhibitors (PD-1, PD-L1 inhibitors) which may aggravate the clinical course of COVID-19. Furthermore, immune side effects such as pneumonitis can be serious and fatal events associated with the use of immune checkpoint inhibitors (ICI) known as immunotherapy and can also be related to COVID-19. As such, concerns emerged around ICI use in patients who test positive for COVID-19, because of potential overlapping clinical complications and immune system enhancement. Only a few studies looked at the outcomes of patients with cancer undergoing active anti-cancer therapy including chemotherapy, radiation therapy, surgery, and ICI with mixed results [7,8,9]. A recent meta-analysis showed that the COVID-19 mortality rate did not significantly differ between patients with cancer whether they were undergoing anti-cancer treatments or not [7].
The safety of using immunotherapy in patients with COVID-19 infections is not well established and mixed results were reported warranting further investigations for better clinical decisions to avoid unnecessary treatment delays that may compromise cancer-related outcomes. Understanding the full scope and impact of ICI treatment in patients with COVID-19 is vital to inform treatment decisions and provide better quality of care. As such, we aimed to look at the mortality rates in patients with cancer treated with immunotherapy who tested positive for COVID-19 in Ontario, Canada.
We conducted a retrospective cohort study in Ontario, Canada’s most populous province with a universal healthcare system using health administrative data at ICES (supplementary 1). ICES is an independent, non-profit research institute whose legal status under Ontario’s health information privacy law allows it to collect and analyze health care and demographic data, without consent, for health system evaluation and improvement. Our study adhered to the RECORD guidelines for health data research [10].
The databases used in our study are described in supplementary Table 1. These datasets were linked using unique encoded identifiers and analyzed at ICES.
Our study included patients with advanced melanoma, lung, bladder, head and neck, kidney or Merkel cell and squamous cutaneous cancers (supplementary Table 2) in Ontario who were treated with immunotherapy between January 2020 and April 2023. Eligible immunotherapy drugs were avelumab, ipilimumab, nivolumab, pembrolizumab, atezolizumab, cemiplimad and durvalumab. Cancer diagnosis was identified from the Ontario Cancer Registry database and treatment with immunotherapy was identified from the Cancer Activity Level Reporting (ALR) and the New Drug Funding Program (NDFP) databases. The cancer sites were comprised of malignancies for which immunotherapy was approved in Ontario at the time of the study. Patients with another cancer diagnosis between starting immunotherapy and 5 years prior to their study diagnosis were excluded to avoid the impact of other systemic therapies for different cancers during the same time. Similarly, patients who did not have a positive COVID-19 test result within 120 days after their immunotherapy treatment were excluded. The codes used for cancer diagnosis and systemic therapy are listed in supplementary Table 3.
The following patient and tumor characteristics were included: age at the time of positive COVID-19 testing, sex, income quintile, rurality, tumor site, disease stage, cancer centre facility level where immunotherapy was given [level 1 being the most complex level of care delivery and service availability (supplementary methods 2) [11]], Charlson Comorbidity Index, Edmonton Symptom Assessment System (ESAS) scores within 60 days prior to COVID-19 testing, laboratory values within 60 days prior to COVID-19 testing [hemoglobin (< or > = 10 g/dl), white blood cells (> or < = 11,000/mm3), platelets ( < = or > 350,000/mm3), lymphocyte count (< or > = 1.1 × 109 ), AST (> or < = 40U/L), ALT (> or > = 40U/L), CRP (> or < = 10 mg/L), albumin (< or > = 35 g/L), calcium (> or < = 2.55mmol/L), creatinine ( < = or > 100umol/l), neutrophil to lymphocyte ratio (NLR), body mass index (BMI)(< or > = 25), and hospital admission and emergency department visits within 60 days prior to COVID-19 testing. Treatment characteristics included prior receipt of chemotherapy within the previous year or radiation therapy within 60 days prior to COVID-19 testing, ICI type (single agent vs. combination therapy), COVID-19 vaccination status (number of doses) and time since immunotherapy initiation. The details on how each variable was operationalized are shown in supplementary Table 4.
The primary outcome of this study was mortality within 30 days of testing positive for COVID-19 as an inpatient or outpatient (index date). We also looked at the clinical factors associated with the 30-day mortality, ICU admissions and ventilation rates within 30 days, and overall survival.
Continuous baseline characteristics for the cohort were summarized using means with standard deviations and medians with interquartile ranges as appropriate; categorical variables were summarized using counts and proportions.
An unadjusted analysis followed by a multivariable logistic regression model were used to evaluate factors associated with the 30-day mortality. A stepwise regression method was used to select variables to include in the final model. A significance level of 0.20 was used to select variables to enter the model and a significance level of 0.25 was used to delete variables from the model. Variables with significant missingness were not considered (> 15%). Adjusted odds ratios (ORs) and 95% confidence intervals were reported. Collinearity and model validity were assessed.
Overall survival was defined as the time from COVID-19 testing until the date of death, censoring on the end of the study period if no death occurred. The probability of survival over time was estimated using Kaplan-Meier (KM) curves.
Throughout, p-values of < = 0.05 were considered statistically significant. To protect patient privacy, cell sizes less than or equal to five are not reported. All analyses were conducted using SAS version 9.4 (SAS Institute, Cary, NC).
Among 14,444 patients with cancer treated with immunotherapy, 281 tested positive for COVID-19 by polymerase chain reaction (PCR) testing within 120 days after treatment and were included in this study (Fig. 1). The median age was 69 years (IQR 62–76), 45% (N = 127) were female and 58% had lung cancer (N = 163). More than half of the patients (62%) were treated in a level 1 or level 2 cancer centre, almost a third (29%) had prior radiation therapy and almost all patients had prior chemotherapy. Almost 60% of patients had a Charlson comorbidity score of 3 or higher and were treated with single agent immunotherapy. The 2 most common immunotherapy drugs used were pembrolizumab and nivolumab. At the time of COVID-19 test, 46 patients (16%) had elevated white blood cells (> 11,000/mm3), 26% had anemia (Hb < 10 g/dl), and 47% had lymphopenia (< 1.1 × 109/L). Furthermore, 57% of patients had an elevated BMI ( > = 25) and a median neutrophil to lymphocyte ratio of 6 (IQR 3–11). The details of patients’ characteristics are presented in Table 1.
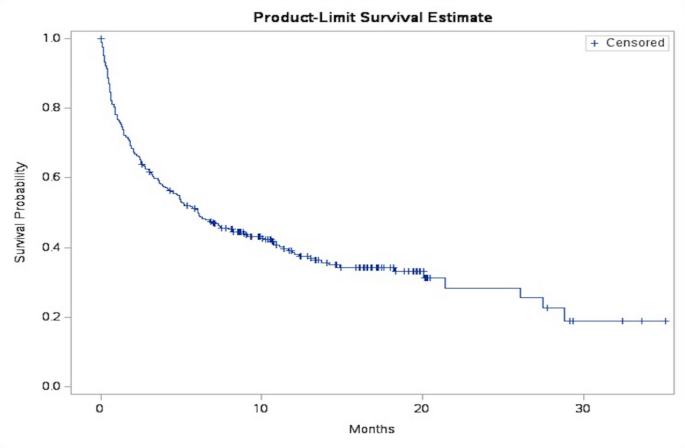
Among 281 eligible patients, 63 (22%) died within 30 days of testing positive for COVID-19. A minority of patients (< 5%) were admitted to the ICU or needed ventilation. The median survival was 6.03 months (95%CI, 1.28–27.5) (Fig. 2).
In the unadjusted models, having a prior emergency department visit, prior hospital admission, prior radiation therapy, de novo stage 4 cancer, low hemoglobin, elevated white blood cells, low albumin levels, and lower NLR were associated with a higher risk of 30-day mortality. Factors associated with a lower risk of mortality were tumor site (lung cancer and head and neck cancer compared to melanoma) and higher income quintile (4 vs. 1). Results from the unadjusted models are presented in Table 2.
The candidate variables considered for the stepwise multivariable logistic regression model were age, sex, income quintile, rurality, tumor site, cancer centre level, Charlson score, prior emergency department visit, prior hospital admission, previous radiation therapy and chemotherapy, hemoglobin, white blood cells, platelets, BMI and immunotherapy drug type. The final adjusted model included age, income quintile, tumor site, hospital admission, prior radiation therapy, hemoglobin, white blood cells and platelets. Older age was associated with a higher risk of 30-day mortality (every additional 10 years, OR 1.60 (95% CI, 1.07–2.39), as were prior hospital admission and prior radiation therapy (OR 1.80, 95% CI 0.86–3.76 and OR 2.40, 95% CI, 1.08–5.28, respectively). Furthermore, lower hemoglobin (Hb < 10 g/dl) and higher white blood cells (> 11,000/mm3) were associated with a higher risk of death (OR 4.08, 95% CI, 1.89–8.82 and OR 3.63, 95%CI, 1.55–8.52, respectively). Higher income quintile (income quintile 4 vs. 1) was associated with a lower risk of mortality (OR 0.24, 95%CI, 0.08–0.74). The adjusted model and the Receiver Operating characteristic (ROC) curve are represented in Table 2; Figs. 3 and 4.
In this population-based study, we report that 22% of patients with cancer treated with immunotherapy in Ontario died within 30 days of testing positive for COVID-19. The mortality rate seems in line with what was reported in the literature. Furthermore, we have shown that certain clinical factors including older age, lower hemoglobin, higher white blood cells, prior radiation therapy and hospital admission were associated with a higher risk mortality. This is in line with previous reports showing that anemia and leukocytosis are worse prognostic factors and previous radiation therapy particularly prior lung irradiation with larger fields [12,13,14]. During and after the pandemic, cancer was shown to be a factor associated with worse survival and increased mortality among patients who tested positive for COVID-19 compared to patients without a cancer diagnosis [15, 16]. This is not surprising as typically patients with cancer are more vulnerable with their immunosuppressive state. The question we tried to address in this study is whether the use of immunotherapy would affect the outcomes in that setting. This is based on some reports showing that a subgroup of patients with COVID-19 may develop a cytokine storm syndrome which is of particular concern in the context of immunotherapy while others show that immunotherapy may interrupt the T cell exhaustion and depletion and augment the immune system response [17, 18]. In a prospective study, 32% of patients with cancer on treatment died within 30 days of their COVID-19 infection [19]. This included any type of treatment for cancer and the study did not have a breakdown of the type of systemic therapy. In another report, mortality was at 38% and most of the patients in this study were not vaccinated [20], unlike our study where most patients were vaccinated. Furthermore, in that study, being on active systemic therapy including chemotherapy and immunotherapy did not increase the risk of mortality compared to patients on no active treatment for solid tumors but the risk was increased in patients with hematologic malignancies [20]. Smaller studies looked at the outcomes in patients treated with immunotherapy and in one report with 61 patients, 24% of patients on immunotherapy treatment died post COVID-19 and this number decreased when adjusted for comorbidities and vaccination status [21]. In another small study, 110 patients on immunotherapy were included and 16% if patients died after having COVID-19 [9]. Overall, the mortality rate does not seem to be higher than most of the reports on mortality in patients with cancer [19,20,21,22,23] while we recognize that some studies showed a lower rate of mortality compared to ours [5, 24]. Furthermore, immunotherapy does not appear to increase the risk of death compared to patients with cancer on other therapies or no therapies.
Some limitations of our study include its retrospective non-comparative nature and our inability to capture the cause of death to determine whether patients’ death was directly related to COVID-19 or to their cancer. Furthermore, key factors such as performance status (ECOG) and COVID-19 symptoms were not captured in our analysis, and we did not look at long term outcomes along with patients’ quality of life which should be considered in future research to provide more comprehensive guidance for clinical treatment. It is also important to mention that testing positive for COVID-19 was based on PCR testing and not rapid antigen testing, and as such, our numbers may be an underestimation of the true numbers we have in Ontario.
Despite some limitations, to our knowledge, our report is one of the largest studies investigating mortality in patients with cancer, treated with immunotherapy, who tested positive for COVID-19. Furthermore, with our model, we were able to adjust for important clinical factors associated with worse mortality including age, and laboratory values (hemoglobin and white blood cells); these factors are necessary for treatment decisions.
Our report was able to provide valuable insights and adds to the body of literature to show that in general patients with cancer treated with immunotherapy do not carry a significantly higher risk of 30-day mortality compared to patients with cancer in general. Learning from this pandemic, one area we can improve is to have real-time data analyses to assess patients’ outcomes and help with treatment decision during the pandemic rather than waiting years before retrospectively reporting the outcomes. As such, the evidence we have supports continuing treating patients with immunotherapy in the event of having COVID-19 infection and treatment cessation or delay is not warranted. Yet, this needs to be cautiously applied as some clinical variables may be associated with a higher risk of mortality. For patients with higher risk, there may be a role for pre-exposure prophylaxis or prevention using approved therapies. Patients’ selection is essential and a risk-benefit assessment with the patient is needed especially that stopping treatment may be detrimental with the disease progression and in patients with potentially long-term benefit. Additionally, the generalizability of the results needs to be carefully considered especially with the rapid changing in the epidemiology of the virus over time and space. Overall, the results from this study and other reports are informative for future pandemics and show the importance of vaccination that is in line with previous reports on the safety of influenza vaccine in patients treated with immunotherapy [25, 26].
Immunotherapy does not seem to increase the risk of 30-day mortality in patients with cancer who test positive for COVID-19. We identified certain clinical factors associated with a higher risk of mortality that need to be carefully considered to help with the treatment decision.
The dataset from this study is held securely in coded form at ICES. While data sharing agreements prohibit ICES from making the dataset publicly available, access can be granted to those who meet pre-specified criteria for confidential access. The full data set creation plan and underlying analytic code are available from the authors upon request, understanding that the programs may rely upon coding templates or macros that are unique to ICES and are therefore either inaccessible or may require modification.
This study was supported by ICES, which is funded by an annual grant from the Ontario Ministry of Health (MOH) and the Ministry of Long-Term Care (MLTC). This study also received funding from the Medical Oncology Research Fund at the Verpseeten Family Cancer centre. This document used data adapted from the Statistics Canada Postal CodeOM Conversion File, which is based on data licensed from Canada Post Corporation, and/or data adapted from the Ontario Ministry of Health Postal Code Conversion File, which contains data copied under license from ©Canada Post Corporation and Statistics Canada. Parts of this material are based on data and/or information compiled and provided by MOH, CIHI, Ontario Health and OCMOH. The analyses, conclusions, opinions and statements expressed herein are solely those of the authors and do not reflect those of the funding or data sources; no endorsement is intended or should be inferred. Furthermore, this study was supported by the Ontario Health Data Platform (OHDP), a Province of Ontario initiative to support Ontario’s ongoing response to COVID-19 and its related impacts. The opinions, results and conclusions reported in this paper are those of the authors and are independent from the funding sources. No endorsement by the OHDP, its partners, or the Province of Ontario is intended or should be inferred.
As a prescribed entity under Ontario’s privacy legislation [Personal Health Information Protection Act (PHIPA)], ICES is authorized to collect and use health care data for the purposes of health system analysis, evaluation, and decision support. Secure access to these data is governed by policies and procedures that are approved by the Information and Privacy Commissioner of Ontario. Section 45 of PHIPA authorizes ICES to collect personal health information, without consent, for the purpose of analysis or compiling statistical information with respect to the management of, evaluation or monitoring of the allocation of resources to or planning for all or part of the health system. Projects that use data collected by ICES under section 45 of PHIPA, and use no other data, are exempt from REB review. As such, the use of the data in this project is authorized under section 45 and approved by ICES’ Privacy and Legal Office.
N/A.
J. Raphael: advisory boards with Merck, Lilly, AstraZeneca and Novartis.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
Raphael, J., Le, B., Singh, S. et al. Early mortality in patients with cancer and COVID-19 infection treated with immunotherapy. BMC Cancer 25, 922 (2025). https://doi.org/10.1186/s12885-025-14318-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12885-025-14318-2