Lipids in Health and Disease volume 24, Article number: 188 (2025) Cite this article
Characterized by intermittent hypoxia (IH), sleep fragmentation, and enhanced sympathetic nervous system activity, obstructive sleep apnea (OSA) precipitates oxidative stress, systemic inflammation, and metabolic perturbations. These disturbances manifest as alterations in serum uric acid (SUA) and high-density lipoprotein cholesterol (HDL-C) levels. Recently, the ratio of SUA to HDL-C (UHR) has emerged as a potential biomarker reflecting both inflammatory and metabolic status. This study investigates the association between UHR and OSA.
Using a cross-sectional design, data were extracted from adults aged 20 years and older in the National Health and Nutrition Examination Survey (NHANES) database, covering the period from 2015 to March 2020. OSA was determined via the NHANES Sleep Disorders Questionnaire. The investigation employed weighted logistic regression alongside trend tests to evaluate the relationship between UHR and OSA. Nonlinear relationships were examined with restricted cubic spline analysis and threshold effect analysis. Receiver operating characteristic (ROC) curves were utilized to compare the predictive capacities of UHR, SUA, and HDL-C for OSA, with the area under the curve (AUC) calculated to assess the models’ predictive accuracy. In addition, mediation analyses were conducted to explore the role of body mass index (BMI) in this association, and sensitivity analyses confirmed the robustness of the findings. Subgroup analyses further assessed the impact of various covariates.
Among the 9985 adults included, 4906 were identified as individuals with OSA. A positive association between UHR and the risk of OSA was observed (OR = 1.02; 95% CI: 1.01, 1.04; P = 0.014). Moreover, a nonlinear relationship was confirmed (P for nonlinearity = 0.024), with an inflection point at a UHR level of 10.23. UHR demonstrated greater predictive accuracy for OSA (AUC = 0.591) compared to SUA (AUC = 0.568) and HDL-C (AUC = 0.580). Additionally, BMI was found to partially mediate the relationship between UHR and OSA, with a mediation proportion of 61.99%. This association remained significant within specific subpopulations (P < 0.05) and was further modulated by factors such as age, alcohol consumption, and diabetes status (P for interaction < 0.05). Sensitivity analyses underscored the stability of these results.
UHR is positively correlated with the risk of OSA in adults, with BMI serving as a partial mediator. The findings support UHR as a viable biomarker for early detection and risk assessment in patients with OSA. Strategies focusing on weight management may reduce the risk of OSA among individuals with elevated UHR levels.
Obstructive sleep apnea (OSA) is a prevalent sleep-related breathing disorder marked by recurrent episodes of upper airway obstruction during sleep. Worldwide, nearly 1 billion adults aged 30–69 suffer from OSA, with an estimated 425 million adults diagnosed with moderate to severe OSA [1]. The economic implications of OSA are substantial, as evidenced by a systematic review from Italy indicating an annual socioeconomic cost ranging from €10.7 billion to €32 billion, with direct healthcare expenditures constituting about 60% of these costs [2]. OSA contributes to increased systemic inflammation, oxidative stress, and metabolic disturbances, thereby establishing a bidirectional link with conditions such as diabetes, hypertension, heart failure, and metabolic syndrome [3,4,5,6]. Intermittent hypoxia (IH) and sleep fragmentation in patients with OSA contribute to insulin resistance, hypertension, and dyslipidemia via mechanisms involving sympathetic activation, inflammatory responses, and oxidative stress. These metabolic abnormalities, in turn, exacerbate the pathogenesis of OSA. For instance, insulin resistance impairs neuromuscular control of the airway, while increased visceral fat leads to narrowing and potential collapse of the airway. This reciprocal relationship between metabolic dysregulation and OSA creates a self-perpetuating cycle [7]. Notably, individuals with OSA, particularly women and those with severe conditions, often suffer significant declines in quality of life, encompassing daily activities, alertness, and social interactions [8]. Hence, timely diagnosis and effective management of OSA are imperative to mitigate its adverse health consequences.
Recent studies have highlighted serum uric acid (SUA) and high-density lipoprotein cholesterol (HDL-C) as key indicators of OSA. The pathogenesis of OSA involves hyperuricemia due to crystal deposition, heightened oxidative stress, and inflammatory activation [9]. OSA-related IH stimulates xanthine oxidase, which in turn elevates SUA levels [10]. Moreover, a Mendelian randomization study highlighted OSA as a contributory factor to hyperuricemia [11]. Conversely, HDL-C is recognized for its anti-inflammatory, antioxidative, and endothelial protective functions, as well as its role in regulating insulin sensitivity and promoting reverse cholesterol transport [12]. HDL-C deficiency exacerbates endothelial dysfunction and atherosclerosis, increasing OSA risk [13]. In turn, OSA can diminish the protective effects of HDL-C, thereby enhancing the risk of endothelial dysfunction and atherosclerosis [14]. Furthermore, a systematic review confirmed that OSA disrupts lipid profiles, particularly by lowering HDL-C levels, which increases the propensity for cardiovascular diseases (CVDs) among affected individuals [15].
While elevated SUA levels reflect increased xanthine oxidase activity under IH conditions and reduced HDL-C levels indicate lipid peroxidation due to IH, these biomarkers individually do not fully represent the complex interplay between pro-oxidant and antioxidant processes in OSA. The ratio of SUA to HDL-C (UHR) has been proposed as a novel, integrated biomarker better capturing the combined effects of oxidative stress and metabolic imbalance. Research has shown that UHR is positively associated with various conditions, including diabetes, chronic kidney disease, non-alcoholic fatty liver disease, and metabolic syndrome, yet its application in OSA research is still emerging [16,17,18,19].
Given these considerations, this study, utilizing data from the National Health and Nutrition Examination Survey (NHANES), posits that UHR is positively associated with OSA, with body mass index (BMI) potentially playing a mediating role. This investigation seeks to substantiate the link between UHR and OSA, thereby providing a valuable tool for early detection and risk assessment in individuals with OSA.
The NHANES employs a sophisticated epidemiological approach to gather representative health and nutritional data for the U.S. population, including adults and children. This comprehensive survey collects demographic, dietary, and health-related data through interviews, physical examinations, and laboratory analysis of biological samples. It utilizes a complex, stratified, multistage probability cluster sampling methodology. All NHANES procedures have been authorized by the Research Ethics Review Board of the National Center for Health Statistics, and all participants have provided written informed consent.
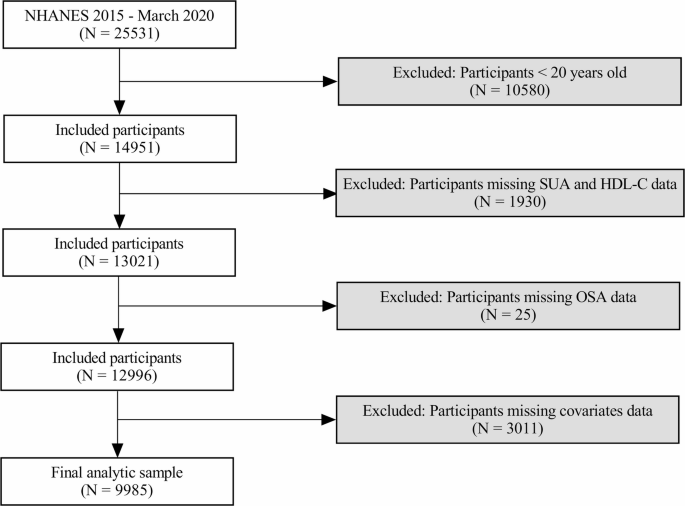
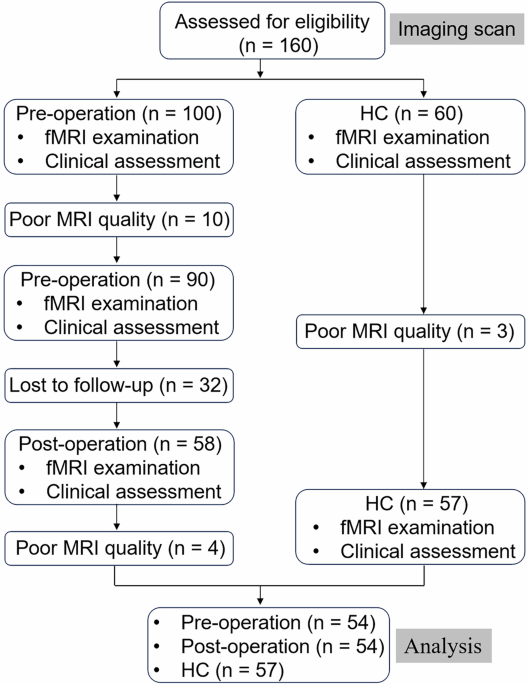
This study used a cross-sectional design, drawing data from the 2015–2016 and 2017-March 2020 NHANES cycles, which included measurements of SUA and HDL-C, as well as questionnaire data related to OSA symptoms. Data were accessible via the NHANES website. Initially, 25,531 individuals were surveyed, with the following exclusion criteria applied: individuals under 20 years of age (n = 10580), those missing SUA and HDL-C data (n = 1930), participants without OSA-related questionnaire responses (n = 25), and those lacking data on relevant covariates (n = 3011). This resulted in a final sample of 9985 eligible adults (Fig. 1).
Quantification of HDL-C was conducted using a standardized enzymatic assay, which involves sequential chemical reactions to isolate and measure HDL-C. This process includes precipitation of non-HDL particles, enzymatic conversion of HDL esters to cholesterol, oxidation to produce hydrogen peroxide, and a chromogenic reaction to form a measurable color change. Absorbance was read spectrophotometrically at 600 nm.
SUA levels were determined using different methodologies across survey cycles. In 2015–2016, a timed endpoint method was employed using an automated analyzer, which involves enzymatic oxidation of uric acid and subsequent colorimetric detection of the reaction product. In the 2017-March 2020 cycle, a modified two-point endpoint method was used, incorporating a chromogenic substrate in the reaction, with measurements taken at 546 nm. The UHR was calculated as follows: UHR (%) = [SUA (mg/dL) / HDL-C (mg/dL)] × 100.
OSA was diagnosed based on responses to the NHANES Sleep Disorders Questionnaire, which probes several domains related to sleep disturbances, including frequency of snoring, breathing interruptions, and daytime sleepiness. OSA was identified if participants reported frequent snoring or breathing interruptions (≥ 3 nights per week), or excessive daytime sleepiness (≥ 16–30 times per month) while maintaining a minimum of 7 h of sleep on weekdays or workdays [20].
In this study, a comprehensive array of covariates was integrated into the analytical model to mitigate potential confounding effects on the relationship between various factors and the occurrence of OSA. The selection of these covariates was guided by previous literature and clinical expertise, ensuring that key influencing variables were adequately controlled [21, 22]. Demographic factors—including age, sex, race, education level, marital status, and poverty-to-income ratio (PIR)—provided a detailed overview of the population’s social and economic context [23]. Lifestyle factors were also meticulously considered, such as BMI stratification, smoking status (categorized as non-smokers, former smokers, and current smokers), alcohol consumption (non-drinkers and moderate drinkers), and physical activity level measured by Metabolic Equivalent of Tasks (MET), which collectively reflect behavioral and health-related patterns that may influence OSA risk [24,25,26,27]. Furthermore, the model adjusted for a range of clinically pertinent comorbidities known to be associated with OSA, including hypertension, diabetes, and CVDs (specifically coronary heart disease, angina, heart failure, and stroke). Respiratory conditions such as asthma and chronic obstructive pulmonary disease (COPD), as well as mental health status assessed via depressive symptoms (Patient Health Questionnaire-9 [PHQ-9] score ≥ 10), were also incorporated [28]. This rigorous adjustment strategy, supported by NHANES data, was designed to enhance the validity and reliability of the findings by addressing the complex interplay among sociodemographic, lifestyle, and health-related factors contributing to OSA. The definition of covariates is detailed in Supplementary Materials.
Statistical analyses were performed in accordance with NHANES’ complex sampling design, incorporating sampling weights, masked variance pseudo-stratum, and masked variance pseudo-cluster to ensure representativeness. The NHANES 2015–2016 cycle covered 2 years, while the NHANES 2017-March 2020 cycle spanned 3.2 years. To harmonize the data, the sampling weights for the NHANES 2015–2016 cycle were adjusted by multiplying them by 2/5.2, and the weights for the 2017-March 2020 cycle were adjusted by multiplying them by 3.2/5.2. This weight adjustment process is detailed in (Supplementary Table S1). Variables were converted into categorical formats and described using frequencies and weighted percentages. Participants were sorted into UHR quartiles (Q1-Q4), and baseline characteristics were compared using the Rao-Scott Chi-Square test. Logistic regression models, both univariate and multivariate, were used to examine UHR’s correlation with OSA. The models were progressively adjusted, from unadjusted (Model 1), adjusting for demographic variables (Model 2), to fully adjusted for all covariates (Model 3). Results were expressed in odds ratios (OR) and 95% confidence intervals (CI). UHR quartiles were also analyzed as continuous variables for trend tests. To probe nonlinear relationships, restricted cubic spline (RCS) analysis was employed after full covariate adjustment, producing RCS curves. If nonlinearity was detected, threshold effect analysis was conducted based on the inflection point. Mediation by BMI was explored using the “mediation” R package, adjusting for a comprehensive set of covariates, and employing the nonparametric percentile Bootstrap method for robust effect size estimation [29]. Additionally, receiver operating characteristic (ROC) curve analyses assessed UHR, SUA, and HDL-C’s predictive performance for OSA, comparing the area under the curve (AUC) using the bootstrap method. Subgroups were analyzed through weighted multivariate regression, incorporating interaction terms to assess heterogeneity. Sensitivity analyses using unweighted logistic regression confirmed the stability of the findings. All analyses were performed using R software version 4.2.3, with a significance threshold set at P < 0.05.
Detailed in Table 1, the study population comprised 51.31% females and 48.69% males, with a total weighted OSA prevalence of 47.48%. UHR levels were stratified into quartiles, with the ranges being 1.36 ≤ Q1 < 7.37, 7.37 ≤ Q2 < 10.22, 10.22 ≤ Q3 < 13.90, and 13.90 ≤ Q4 ≤ 80.0. There were significant differences in UHR across demographic and clinical characteristics. Individuals in the highest UHR quartile (Q4) were predominantly male, had lower levels of educational attainment, higher rates of obesity, lower to middle income, and were more likely to smoke and less likely to drink. Additionally, they had higher incidences of hypertension, diabetes, and cardiovascular diseases (CVDs). Significant variations in race and marital status were also noted across the UHR quartiles (P < 0.05).
Table 2 summarizes the logistic regression analyses that evaluated the association between UHR and the risk of OSA. In the unadjusted Model 1, UHR exhibited a robust and statistically significant positive association with OSA risk (P < 0.001), indicating that higher UHR levels correlated with increased odds of OSA. This relationship persisted even after adjusting for key demographic and socioeconomic variables—including age, sex, race, educational attainment, marital status, and PIR—in Model 2 (P < 0.001). Further adjustment for additional potential confounders in the fully adjusted Model 3 consistently confirmed the independent association between UHR and OSA risk.
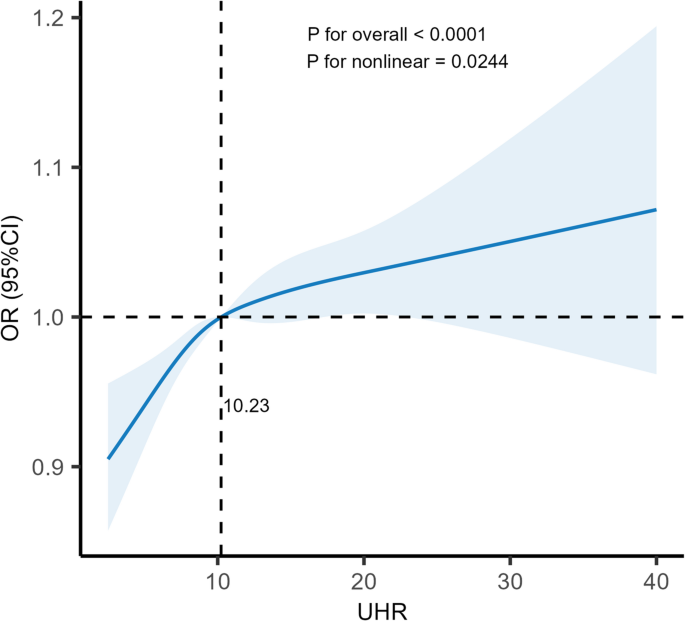
RCS analysis further investigated the potential nonlinear relationship between UHR and OSA risk. As illustrated in Fig. 2, the analysis revealed a significant nonlinear association, with an inflection point identified at a UHR level of 10.23 (P for nonlinearity = 0.024). Specifically, below this threshold, the risk of OSA increased markedly with rising UHR levels (P = 0.018). However, once UHR exceeded the inflection point, the strength of this association diminished, and the risk plateaued, with the OR no longer reaching statistical significance (P = 0.10) (Table 3). These findings suggest the existence of a potential threshold effect, where the association may be more pronounced at lower to moderate levels but attenuates beyond the identified inflection point.
RCS analysis of UHR’s association with OSA. Odds ratio (solid line) and 95% confidence intervals (transparent area) were adjusted based on Model 3
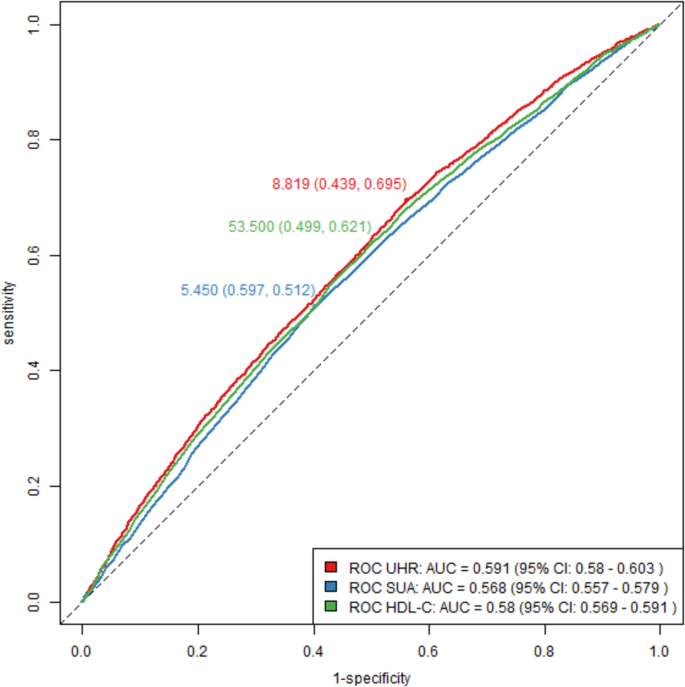
As shown in Fig. 3, UHR demonstrated slightly superior discriminatory ability compared to SUA and HDL-C. The AUC for UHR was 0.591, marginally higher than the AUCs for SUA (0.568) and HDL-C (0.580), with the difference reaching statistical significance (P < 0.01). The detailed cutoff values, sensitivity, specificity, and corresponding Youden index values for each parameter are summarized in Table 4. These results suggest that while UHR shows better predictive value than SUA or HDL-C alone, its overall predictive capacity for OSA remains modest.
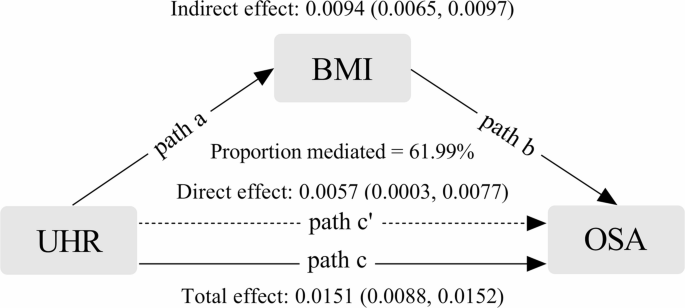
Further analysis examined the potential mediating role of BMI in the relationship between UHR and OSA risk. Notably, obesity rates were significantly higher in participants within the highest UHR quartile (Q4), implying that BMI might partially account for the observed association. Mediation analysis, adjusted for relevant covariates, confirmed that BMI indeed served as a partial mediator in this relationship. The mediation effect accounted for 61.99% of the total association between UHR and OSA risk, with the mediation proportion reaching statistical significance (P < 0.01) (Fig. 4). These findings highlight the substantial role of obesity in mediating the link between elevated UHR levels and increased OSA risk, underscoring the complex interplay between metabolic factors and sleep-disordered breathing.
Path diagram of the mediated analysis model. UHR was defined as the independent variable; OSA as the dependent variable; and BMI as the mediating variable. Path a: the effect of UHR on BMI. Path b: the effect of BMI on OSA. Path c: the total effect of UHR on OSA. Path c’: the direct effect of UHR on OSA that is not mediated by BMI
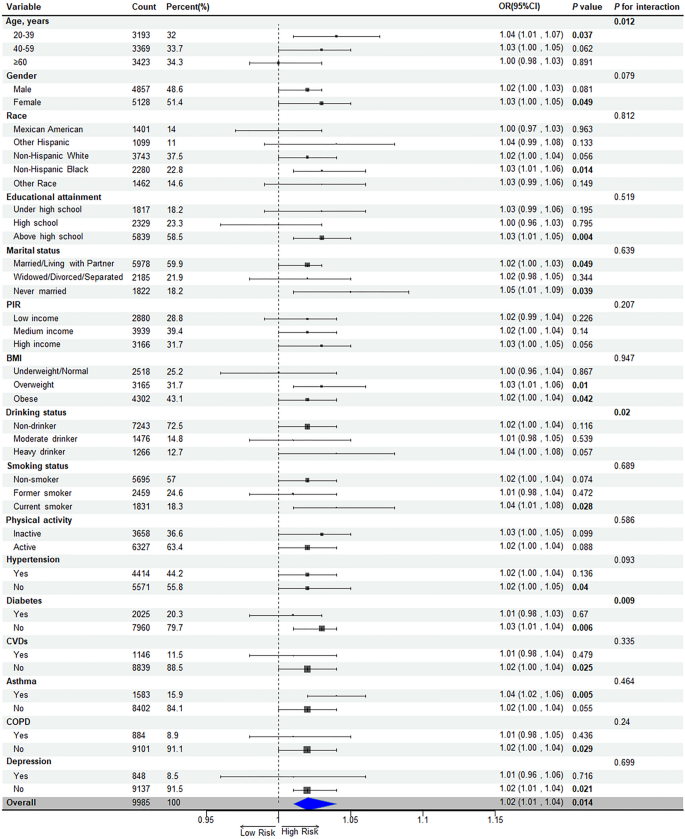
Subgroup analyses explored the relationship between UHR and OSA across various demographic and clinical contexts, showing a consistent positive association across all subgroups. This association was notably significant in younger adults (20–39 years), females, non-Hispanic blacks, those with more never married or cohabitating individuals, overweight or obese individuals, current smokers, and those without diabetes, hypertension, or CVDs (P < 0.05). Age, alcohol consumption, and diabetes status were identified as modifiers of the association strength (P for interaction < 0.05) (Fig. 5).
Consistent with primary findings, sensitivity analysis reinforced the robust positive correlation between UHR and OSA in the fully adjusted model (P < 0.001), with heightened OSA risk observed in the highest UHR quartile (Q4) (P < 0.001) (Supplementary Table S2). These findings underscore the stability and consistency of the observed associations between UHR and OSA across different analytical conditions.
The objective of this study was to investigate the correlation between the UHR and the risk of OSA in adults. Adjustments for relevant covariates showed that each unit increase in UHR was associated with a 2% increase in the risk of OSA. The mechanisms linking UHR to OSA are multifaceted, involving both components of the UHR index, SUA, and HDL-C.
OSA is influenced by mechanical and functional factors. Mechanical factors include anatomical abnormalities that lead to upper airway collapse due to soft tissue deposition and abnormal bone structures [30]. Functionally, the reduction in muscle tone of the upper airway during sleep compromises its ability to remain open, thus heightening the risk of collapse. This is compounded by a reduction in the central respiratory drive, diminishing neuromuscular responsiveness and airway stability [31]. Individuals with OSA often have a lowered respiratory arousal threshold, causing them to awaken in response to minor respiratory disturbances. This disrupts sleep continuity, impairing both sleep quality and physiological recovery [32]. IH, a hallmark of OSA, triggers oxidative stress and systemic inflammation, which can lead to endothelial dysfunction, increased sympathetic activity, and insulin resistance [33]. In conditions of hypoxia, pathways including hypoxia-inducible factor-1α (HIF-1α) and nuclear factor kappa-B (NF-κB) are activated, promoting the generation of reactive oxygen species (ROS) that damage cells by altering proteins, lipids, and nucleic acids [34]. Fluid dynamics also play a role in OSA. Nocturnal rostral fluid shift, where fluid accumulates in the legs during the day and shifts to the upper body when lying down, increases neck circumference and pressure, predisposing individuals to airway collapse [35].
From a clinical perspective, hyperuricemia has been shown to induce oxidative stress, leading to vascular endothelial damage and inflammatory responses [36]. Such pathological changes are particularly relevant in OSA, where nocturnal events exacerbate IH and reoxygenation cycles [37]. Hyperuricemia stimulates inflammatory signaling pathways and the secretion of cytokines like interleukin-1β (IL-1β), interleukin-6 (IL-6), and tumor necrosis factor-α (TNF-α). These inflammatory mediators contribute to systemic inflammation and may exacerbate airway obstruction by promoting mucosal edema [38]. Hyperuricemia also contributes to metabolic dysregulation, exacerbating insulin resistance, dyslipidemia, and obesity, which in turn may increase apnea severity through unstable ventilatory control and pharyngeal fat deposition [39]. OSA-related IH, oxidative stress, inflammation, increased sympathetic activity, and altered hormone production can disrupt the function of lipoprotein lipase, reducing the clearance of circulating lipids and leading to dyslipidemia [40]. Oxidative stress enzymes associated with OSA produce dysfunctional oxidized HDL-C, leading to impaired cholesterol efflux and reduced anti-inflammatory properties [41]. Dyslipidemia contributes to endothelial dysfunction and atherosclerosis, further increasing OSA risk [42].
When UHR was less than 10.23, a 7% increase in OSA risk was observed for each unit increase in UHR (P for nonlinear < 0.05); when UHR was ≥ 10.23, the association was not statistically significant. This finding suggests that below the inflection point, UHR appears more strongly associated with OSA risk; while exceeding the inflection point, the action of UHR may reach a state of saturation, and the body may achieve equilibrium through compensatory mechanisms, probably involving antioxidant enzymes and anti-inflammatory responses.
BMI mediates the impact of UHR on OSA by 61.99%, underscoring obesity as a pivotal intermediary. Established associations link obesity with OSA through anatomical constraints, inflammation, and metabolic disruptions. Obesity leads to fat accumulation around the neck and pharynx, directly compressing the upper airway [43]. Increased adiposity in the tongue impairs its functionality [44]. Fat accumulation in the thoracic and abdominal regions reduces lung capacity and the longitudinal traction of the upper airway, heightening the risk of airway collapse [45]. Adipose tissue secretes TNF-α and IL-1β, which inhibit pharyngeal neuromuscular activity and disrupt ventilatory control, while OSA-associated nocturnal hypoxia and sleep fragmentation further amplify systemic inflammation and oxidative stress, creating a detrimental cycle of “obesity-inflammation-OSA” [46]. Elevated UHR levels correlate with metabolic disturbances, visceral fat accumulation, and increased BMI, contributing to OSA development. Although BMI is a recognized factor in OSA pathogenesis, other pathways, such as oxidative stress and systemic inflammation, may also influence the relationship between UHR and OSA as confounders or independent mediators. Future studies should investigate these interactions for a more comprehensive understanding of the underlying mechanisms.
Although the overall predictive accuracy remained modest (AUC = 0.591), UHR showed marginally better predictive effectiveness for OSA compared to SUA and HDL-C. This emphasizes the complexity of OSA development, which involves multiple factors including oxidative stress, inflammatory responses, metabolic dysfunction, and anatomical elements, not entirely captured by UHR alone. Integrating UHR with other anthropometric and clinical metrics in predictive models could enhance the accuracy of assessing OSA risk.
The relationship between UHR and OSA was predominantly noted in specific demographics including females aged 20–39, non-Hispanic blacks, individuals with at least a high school education, those who have never experienced widowhood/divorce/separation, overweight or obese individuals, current smokers, patients with a history of asthma, and those without diabetes, hypertension, CVDs, or depression. This study explored potential explanations for these subgroup variations. Younger females, less likely to have confounding cardiometabolic conditions, reveal a clearer association between UHR and OSA [47]. Estrogen, known for its protective effects against oxidative stress and inflammation, plays a role in OSA pathophysiology [48, 49]. Aging and menopause, associated with reduced estrogen levels and increased comorbidities, may obscure the UHR-OSA link in older age groups. Non-Hispanic blacks may experience enhanced effects of UHR due to genetic predispositions and healthcare disparities that aggravate untreated OSA [50]. Higher educational attainment correlates with improved health literacy, potentially leading to earlier recognition and diagnosis of OSA symptoms [51]. Individuals receiving high school education or higher may adopt healthier lifestyles, therefore reducing the confounding effects of other risk factors [52]. Marital stability might reflect stronger social support, reducing psychological stress and enhancing sleep quality, thus revealing UHR’s independent effect on OSA [53]. Shared mechanisms between asthma and OSA, like nocturnal hypoxia, may amplify UHR’s impact [54]. Smoking increases oxidative burden while decreasing HDL-C levels [55, 56]. Comorbid conditions such as diabetes, hypertension, or CVDs might mask the UHR-OSA link through competing pathways like insulin resistance and arteriosclerosis [57]. Depression could disrupt the UHR-OSA relationship through alterations in sleep architecture [58]. Significantly, age, diabetes, and drinking status were identified as notable modifiers of the UHR-OSA relationship (P < 0.05), meriting further investigation. Given the risk of multiple comparisons and type I errors, these results warrant validation in subsequent studies.
This research introduces several considerable strengths. It represents the inaugural study investigating the correlation between UHR and the risk of OSA, offering clinically significant findings. As an economical and easily accessible blood biomarker, UHR demonstrates a superior predictive capability relative to SUA and HDL-C. This advantage positions it as an ideal tool for preliminary screening in high-risk populations, promoting early detection and assessment of OSA risk. Crucially, a UHR value below 10.23 shows a strong association with increased OSA risk, implying that early interventions could be particularly effective. Additionally, the mediating role of BMI accentuates the relevance of weight management in mitigating UHR-related OSA risk. The findings also underscore the necessity for customized interventions in specific subgroups, such as young women, smokers, and individuals with obesity. Modifying UHR through pharmaceutical and lifestyle changes could represent a novel approach in OSA management.
However, this study is not without limitations. The cross-sectional design, while utilizing the nationally representative NHANES dataset for hypothesis formulation, does not permit causal inferences. The absence of polysomnography (PSG) data in NHANES means that OSA diagnosis relies on self-reported symptoms through the STOP questionnaire. While validated for population-level screening, this method lacks the sensitivity and specificity of PSG, potentially leading to misclassification bias. That’s to say, asymptomatic OSA cases may be underdiagnosed, while non-OSA individuals with overlapping symptoms could be misclassified. Additionally, NHANES lacks data on dietary patterns (e.g., purine-rich or high-cholesterol diets affecting SUA and HDL-C) or sleep duration and quality, which may independently influence OSA risk. Medication use (e.g., diuretics and xanthine oxidase inhibitors elevating SUA, statins altering HDL-C), genetic variants influencing uric acid metabolism (e.g., SLC2A9 polymorphisms) or lipid regulation (APOE alleles) and environmental exposures to air pollution or occupational hazards (e.g., particulate matter, chemical solvents) may confound the UHR-OSA association. Such factors were not captured. Future research employing methodologies like propensity score matching or machine learning could address these limitations and enhance the study’s robustness. Further prospective interventional trials are anticipated to assess the impact of therapies that lower SUA or enhance HDL-C on OSA.
The analysis, based on a representative sample from NHANES, identifies a positive association between UHR levels and OSA risk in adults, with BMI accounting for 61.99% of this relationship.
These insights have profound clinical implications. Given its cost-effectiveness and accessibility, UHR could significantly aid in the early detection and assessment of OSA risk, especially in settings where PSG is unavailable. Personalized strategies for weight reduction, incorporating dietary changes and increased physical activity, could lessen OSA risk among patients with elevated UHR.
Future studies are encouraged to evaluate the effectiveness of interventions that lower UHR, such as therapies reducing SUA or boosting HDL-C levels. Research should also explore the combined effects of UHR modulation and weight management on therapeutic outcomes. Integrating UHR with existing clinical prediction models or wearable sleep monitoring technologies might improve screening accuracy for OSA in primary care. By translating these findings into practical strategies, UHR has the potential to become a fundamental element in the prevention of OSA and precision medicine, ultimately alleviating the worldwide burden of OSA-related health issues.
No datasets were generated or analysed during the current study.
- OSA:
-
Obstructive sleep apnea
- IH:
-
Intermittent hypoxia
- SUA:
-
Serum uric acid
- HDL-C:
-
High-density lipoprotein cholesterol
- CVDs:
-
Cardiovascular diseases
- UHR:
-
The ratio of serum uric acid to high-density lipoprotein cholesterol
- NHANES:
-
National Health and Nutrition Examination Survey
- BMI:
-
Body mass index
- PIR:
-
Poverty-to-income ratio
- MET:
-
Metabolic Equivalent of Tasks
- COPD:
-
Chronic obstructive pulmonary disease
- PHQ-9:
-
Patient Health Questionnaire-9
- OR:
-
Odds ratio
- CI:
-
Confidence interval
- RCS:
-
Restricted cubic spline
- ROC:
-
Receiver operating characteristic
- AUC:
-
Area under the curve
- HIF-1α:
-
Hypoxia-inducible factor-1α
- NF-κB:
-
Nuclear factor kappa-B
- ROS:
-
Reactive oxygen species
- IL-1β:
-
Interleukin-1β
- IL-6:
-
Interleukin-6
- TNF-α:
-
Tumor necrosis factor-α
- PSG:
-
Polysomnography
We thank the NHANES project and its members.
This research received no external funding.
The National Center for Health Statistics Ethics Review Board authorized the NHANES protocols. All participants provided written informed consent to participate in this study.
The authors declare no competing interests.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
Tang, Y., Liu, J., Zhang, J. et al. Association of serum uric acid-to-high-density lipoprotein cholesterol ratio with obstructive sleep apnea: a cross-sectional study. Lipids Health Dis 24, 188 (2025). https://doi.org/10.1186/s12944-025-02604-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12944-025-02604-8