BMC Pulmonary Medicine volume 25, Article number: 256 (2025) Cite this article
Chronic obstructive pulmonary disease (COPD) involves systemic inflammation and is often accompanied by comorbidities. Blood eosinophil count (BEC) is a key marker of airway inflammation, used for patient stratification and treatment guidance. However, the association between BEC (< 300 vs. ≥ 300 cells/µL) and pulmonary or extrapulmonary comorbidities in COPD remains unclear.
This study analyzed COPD patients from the 2013–2018 National Health and Nutrition Examination Survey (NHANES). Weighted multivariable logistic regression models examined associations between BEC and comorbidities, adjusting for potential confounders. Associations were quantified using odds ratios (ORs) and 95% confidence intervals (CIs).
A total of 614 COPD patients were included (395 with BEC < 300 cells/µL, 219 with BEC ≥ 300 cells/µL). Patients with BEC ≥ 300 cells/µL had a higher prevalence of extrapulmonary comorbidities than those with BEC < 300 cells/µL (85.35% vs. 71.48%). Adjusted analysis showed that BEC ≥ 300 cells/µL was significantly associated with increased odds of any extrapulmonary comorbidity (OR = 2.03, 95% CI: 1.19–3.44, p = 0.009), congestive heart failure (OR = 1.69, 95% CI: 1.02–2.82, p = 0.043), and renal dysfunction (OR = 1.95, 95% CI: 1.01–3.79, p = 0.048), but not with pulmonary comorbidities. Sensitivity analyses using 3- and 4-level BEC categories revealed consistent trends, with higher BEC levels significantly associated with greater prevalence of at least one extrapulmonary comorbidity.
In COPD, higher BEC (≥ 300 cells/µL) is significantly associated with extrapulmonary comorbidities, particularly congestive heart failure and renal dysfunction, but not pulmonary comorbidities.
Chronic obstructive pulmonary disease (COPD), a lung disease marked by ongoing inflammation and restricted airflow, stands as the third major cause of mortality globally [1,2,3]. Beyond its pulmonary manifestations, COPD is associated with systemic inflammation that drives multiple comorbid conditions, including cardiovascular diseases, metabolic disorders, and renal dysfunction [4,5,6]. These comorbidities substantially increase the overall disease burden and mortality [4,5,6].
Eosinophils, a subtype of white blood cell involved in immune regulation, have emerged as important biomarkers and potential mediators of COPD pathogenesis [7]. An elevated blood eosinophil count (BEC), often defined as ≥ 300 cells/µL, has been widely adopted in clinical practice to guide anti-inflammatory therapy. This cutoff was selected based on its established clinical significance and previous research linking it to COPD exacerbations and therapeutic responses. Specifically, patients with BEC ≥ 300 cells/µL have been shown to exhibit a higher likelihood of acute exacerbations, a better response to corticosteroid therapy, and heightened systemic inflammation [2, 8, 9]. Furthermore, the NOTUS trial showed that dupilumab, a biologic targeting IL-4Rα, improved lung function and reduced exacerbations in COPD patients with BEC ≥ 300 cells/µL [10, 11]. However, such therapies are indicated only for a small subset of patients with frequent exacerbations despite triple therapy and with type 2 inflammation, limiting their broader applicability.
However, important knowledge gaps remain regarding the systemic impact of BEC in COPD. Although eosinophilic inflammation in the airways correlates with COPD severity [12], it is not yet clear whether elevated blood eosinophil counts are associated with the presence or severity of pulmonary and extrapulmonary comorbidities. Mechanistic studies have suggested that eosinophils might contribute to cardiovascular disease progression by promoting endothelial dysfunction and plaque destabilization [13]; however, evidence supporting this effect in COPD populations is limited. Similarly, eosinophil-derived cytokines (such as IL-5, IL-4, and IL-13) have been implicated in metabolic dysregulation and organ fibrosis, yet the clinical significance of these pathways in the context of COPD comorbidities remains unproven [14, 15]. To address these gaps, a nationally representative dataset– the National Health and Nutrition Examination Survey (NHANES)– offers an opportunity to examine these associations at a population level. Clarifying the association between BEC and COPD-related comorbidities could yield new insights into the systemic consequences of eosinophilic inflammation and help inform comorbidity-focused treatment strategies, particularly as biologic therapies for COPD become more prominent [10, 11, 16].
Therefore, we conducted this cross-sectional study utilizing data from a nationally representative cohort (the NHANES 2013–2018) to evaluate the association between BEC (< 300 cells/µL vs. ≥ 300 cells/µL) and both pulmonary and extrapulmonary comorbidities among patients with COPD.
This cross-sectional study using data from three consecutive NHANES cycles (2013–2014, 2015–2016, and 2017–2018), a nationally representative survey designed to assess the health status and nutritional condition of the non-institutionalized U.S. population. To achieve national representativeness, the NHANES survey utilizes a multi-stage, stratified cluster sampling design with oversampling of minority populations. The study protocol was approved by the National Center for Health Statistics (NCHS) Research Ethics Review Board, and all participants provided written informed consent (details available at: https://wwwn.cdc.gov/nchs/nhanes/).
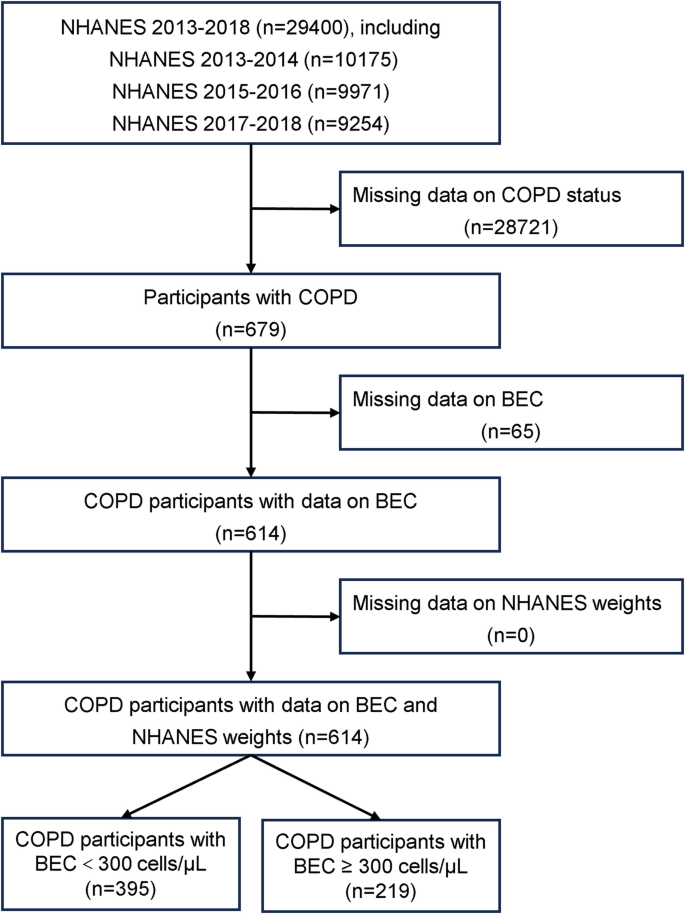
COPD was defined based on self-reported physician diagnosis. Of the 29,400 NHANES participants from 2013 to 2018, individuals with missing data on COPD status, BEC, key covariates, or survey weights were excluded. The final study cohort comprised 614 COPD patients, categorized into two groups: 395 (64.3%) with BEC < 300 cells/µL and 219 (35.7%) with BEC ≥ 300 cells/µL (Fig. 1).
Study population selection flowchart from NHANES. BEC, blood eosinophil count; NHANES, National Health and Nutrition Examination Survey; COPD, chronic obstructive pulmonary disease
The exposure variable was BEC, categorized as < 300 cells/µL or ≥ 300 cells/µL. The 300 cells/µL threshold was selected based on its established clinical relevance and prior studies linking this cutoff to COPD exacerbations and therapeutic responses [2, 10, 11]. Venous blood samples were collected and analyzed following standardized laboratory protocols. In NHANES, BEC is measured only once per participant at the time of examination, so our analysis was based on a single measurement. NHANES variable codes for BEC are detailed in Additional file 1: Table S1.
The study outcomes included both pulmonary and extrapulmonary comorbidities. Pulmonary comorbidities comprised any pulmonary condition, asthma, chronic bronchitis, emphysema, and lung cancer. Extrapulmonary comorbidities encompassed any extrapulmonary condition, congestive heart failure, hypercholesterolemia, coronary heart disease, diabetes, heart attack, and renal dysfunction. Comorbidity data were derived from NHANES questionnaire responses, based on self-reported physician-diagnosed conditions. The definitions and corresponding NHANES variable codes are provided in Additional file 1: Table S1.
Covariates were selected based on prior literature and their potential associations with BEC or comorbidity outcomes. In addition to age, sex, race/ethnicity, body mass index (BMI), and smoking exposure, we adjusted for socioeconomic indicators including marital status, education level, and annual household income, given their documented links to health behaviors, access to care, and chronic disease burden [17]. Data were extracted from NHANES demographic, examination, and questionnaire modules, with detailed variable definitions and NHANES codes available in Additional file 1: Table S1.
As a supplementary analysis, we further stratified BEC into 3-level (< 300, 300–599, ≥ 600 cells/µL) and 4-level (< 100, 100–149, 150–299, ≥ 300 cells/µL) categories to explore potential trends in the association with comorbidities. We calculated the prevalence of having at least one pulmonary or extrapulmonary comorbidity within each subgroup and assessed trends across BEC strata using survey-weighted logistic regression models adjusted for all covariates.
All statistical analyses adhered to NHANES complex survey design guidelines. To account for non-response and post-stratification adjustments, sampling weights (WTMEC6YR = WTMEC2YR × 1/3) were applied, ensuring nationally representative estimates. Continuous variables were reported as weighted means ± standard deviations (SD), while categorical variables were expressed as weighted proportions. Weighted multivariable logistic regression models were employed to assess the associations between BEC categories (≥ 300 cells/µL vs. < 300 cells/µL) and comorbidities using a hierarchical approach: Model 1: Unadjusted analysis; Model 2: Adjusted for sex, age, and race or ethnicity; Model 3: Fully adjusted for all covariates; Associations were quantified using odds ratios (ORs) alongside 95% confidence intervals (CIs). In the sensitivity analyses, survey-weighted logistic regression models were used to assess linear trends across ordered BEC categories. A two-tailed p-value < 0.05 was considered statistically significant. All statistical analyses were conducted using Stata 17.0, following the STrengthening the Reporting of OBservational studies in Epidemiology (STROBE) guidelines [18].
Table 1 provides an overview of the baseline characteristics for a cohort of 614 patients diagnosed with COPD. Specifically, 395 patients (64.33%) had a BEC < 300 cells/µL and 219 (35.67%) had a BEC ≥ 300 cells/µL. The two groups were similar in age (mean 64.73 vs. 64.24 years, p = 0.632), sex distribution (p = 0.792), and race/ethnicity (p = 0.154). Non-Hispanic White individuals constituted 71.39% of the BEC ≥ 300 group and 64.02% of the BEC < 300 group (p = 0.154). Participants with BEC ≥ 300 cells/µL had a significantly lower mean body mass index (BMI) than those with BEC < 300 (29.35 vs. 31.32 kg/m², p = 0.008). In addition, the proportion of participants with BMI ≥ 25 kg/m² (overweight or obese) was significantly higher in the BEC ≥ 300 group than in the BEC < 300 group (77.87% vs. 69.18%, p = 0.001). The prevalence of current smoking was also lower in the BEC ≥ 300 group compared to the BEC < 300 group (39.88% vs. 50.79%, p = 0.037). There were no significant differences between the two groups in marital status, educational level, or household income (p > 0.05).
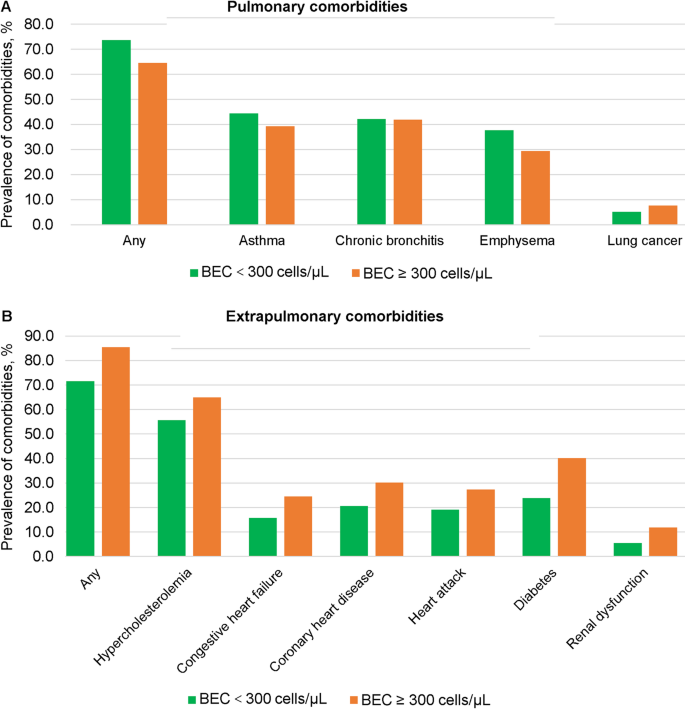
As shown in Table 1 and Fig. 2, participants with BEC ≥ 300 cells/µL exhibited numerically lower prevalence rates of pulmonary comorbidities compared to the BEC < 300 cells/µL group, including any pulmonary comorbidity (64.64% vs. 73.58%), asthma (39.21% vs. 44.36%), chronic bronchitis (41.88% vs. 42.23%), and emphysema (29.41% vs. 37.72%), though none of these differences reached statistical significance (p > 0.05). Notably, lung cancer prevalence was non-significantly higher in the BEC ≥ 300 cells/µL group (7.64% vs. 5.13%, p > 0.05).
Prevalence comparison of comorbidities in COPD participants across varying blood eosinophil levels. A, Pulmonary comorbidities. B, Extrapulmonary comorbidities. BEC, blood eosinophil count; COPD, chronic obstructive pualmonary disease
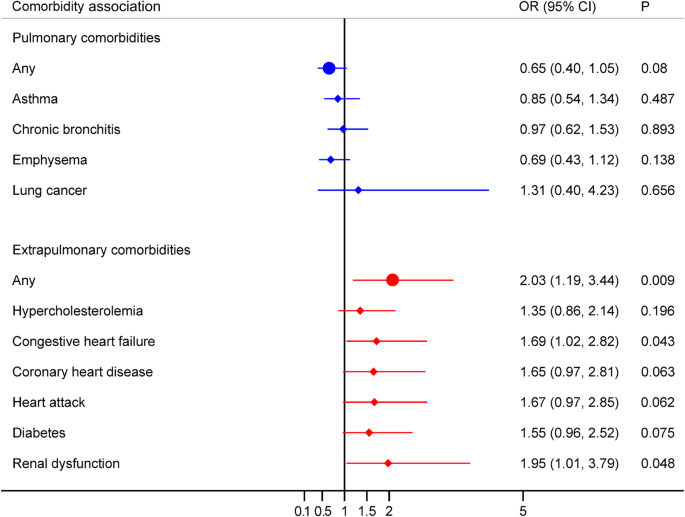
Table 2 summarizes the association between BEC and pulmonary comorbidities in COPD patients. In the unadjusted model, no statistically significant association was observed between higher BEC (≥ 300 cells/µL) and any pulmonary comorbidity (OR = 0.63, 95% CI:0.38–1.04, p = 0.069). This non-significant association persisted in the minimally adjusted model (OR = 0.64, 95% CI:0.39–1.06, p = 0.082) and fully adjusted model (OR = 0.65, 95% CI:0.40–1.05, p = 0.080). Notably, fully adjusted analyses revealed no significant associations between BEC and specific pulmonary comorbidities: asthma: (OR = 0.85, 95% CI:0.54–1.34, p = 0.487); chronic bronchitis: (OR = 0.97, 95% CI:0.62–1.53, p = 0.893); emphysema: (OR = 0.69, 95% CI:0.43–1.12, p = 0.138); lung cancer: (OR = 1.31, 95% CI:0.40–4.23, p = 0.656).These findings are further visualized in Fig. 3; Additional file 1: Figure S1.
Associations between BEC category (≥ 300 vs. < 300 cells/µL) and comorbidities in COPD participants, fully adjusted model. BEC, blood eosinophil count; COPD, chronic obstructive pulmonary disease; OR, odds ratio; CI, confidence interval
As shown in Table 1 and Fig. 2, participants with BEC ≥ 300 cells/µL exhibited a significantly higher prevalence of extrapulmonary comorbidities compared to those with BEC < 300 cells/µL. Specifically, 85.35% of individuals in the high BEC group had at least one extrapulmonary comorbidity, versus 71.48% in the low BEC group. The prevalence of specific comorbid conditions was also significantly higher in the high BEC group, including hypercholesterolemia (65.02% vs. 55.63%), congestive heart failure (24.46% vs. 15.85%), coronary heart disease (30.22% vs. 20.59%), heart attack (27.43% vs. 19.12%), diabetes (40.12% vs. 23.85%), and renal dysfunction (11.83% vs. 5.47%).
Table 2 presents the associations between blood eosinophil count and extrapulmonary comorbidities in COPD patients. In contrast to pulmonary comorbidities, BEC ≥ 300 cells/µL was significantly associated with greater odds of having any extrapulmonary comorbidity in the unadjusted model (OR = 2.19, 95% CI: 1.31–3.67; p = 0.003). This association remained significant after adjusting for potential confounders, including in a minimally adjusted model (OR = 2.20, 95% CI: 1.30–3.71; p = 0.003) and a fully adjusted model (OR = 2.03, 95% CI: 1.19–3.44; p = 0.009). In fully adjusted analyses, BEC ≥ 300 cells/µL was also significantly associated with higher odds of congestive heart failure (OR = 1.69, 95% CI: 1.02–2.82; p = 0.043) and renal dysfunction (OR = 1.95, 95% CI: 1.01–3.79; p = 0.048). However, a significant association with diabetes was present in the unadjusted model (OR = 1.90, 95% CI: 1.17–3.09; p = 0.009), but this association was attenuated and lost significance after full adjustment (OR = 1.55, 95% CI: 0.96–2.52; p = 0.075). No significant associations were found between BEC and hypercholesterolemia, coronary heart disease, or heart attack in these models (p > 0.05), as shown in Fig. 3; Additional file 1: Figure S2.
Sensitivity analyses showed that the prevalence of having at least one extrapulmonary comorbidity increased progressively with higher BEC levels, while the prevalence of at least one pulmonary comorbidity decreased. These trends were statistically significant in both 3-level (p for trend = 0.015 and 0.025 for extrapulmonary and pulmonary comorbidities, respectively) and 4-level (p for trend = 0.017 and 0.023, respectively) BEC analyses (Additional file 1: Tables S2, S3; Figure S3).
In this nationally representative study based on NHANES 2013–2018 data, we examined how BEC dichotomized at 300 cells/µL relates to the prevalence of pulmonary and extrapulmonary comorbidities in individuals with COPD. Notably, COPD participants with elevated BEC (≥ 300 cells/µL) had a consistently higher prevalence of extrapulmonary comorbidities compared to their counterparts with lower BEC (< 300 cells/µL), whereas the prevalence of pulmonary comorbidities did not significantly differ between these groups. Furthermore, in multivariable analyses, a higher BEC (≥ 300 cells/µL) was significantly associated with greater odds of having extrapulmonary comorbidities—most prominently congestive heart failure and renal dysfunction—while no significant associations were observed between BEC and any pulmonary comorbidity. These observations underscore the systemic implications of eosinophilic inflammation in COPD and suggest a potential mechanistic role for eosinophils in the development of comorbidities outside the lungs. To our knowledge, this study represents the first population-based analysis to comprehensively assess comorbidity patterns stratified by BEC thresholds in a nationally representative COPD cohort, and it provides new insights that could guide clinical strategies for managing comorbidities in COPD.
The mechanisms underlying the distinct associations between BEC and COPD-related comorbidities (pulmonary versus extrapulmonary) are not yet fully understood. In our analysis, BEC was not significantly associated with any of the evaluated pulmonary comorbidities, suggesting that eosinophilic inflammation may not be a major pathogenic driver of structural lung diseases (e.g., emphysema or chronic bronchitis) in stable COPD [8]. By contrast, having an elevated eosinophil count (≥ 300 cells/µL) was strongly associated with certain extrapulmonary comorbidities—especially congestive heart failure and renal dysfunction—in alignment with emerging evidence implicating eosinophils in systemic inflammatory pathways. One potential explanation is that eosinophils contribute to systemic pathology by promoting endothelial dysfunction and atherosclerotic plaque instability through the release of cytotoxic mediators and pro-inflammatory cytokines. These mechanisms are known to accelerate cardiovascular remodeling and organ fibrosis in preclinical models [19,20,21,22]. It is also noteworthy that we observed a marginal association between higher BEC and diabetes (p = 0.075 in fully adjusted models), hinting at the involvement of eosinophil-derived cytokines in metabolic dysregulation. Although this association did not reach statistical significance, the trend warrants further investigation to elucidate the underlying mechanisms.
Our findings extend prior evidence regarding the systemic effects of eosinophilic inflammation in COPD by indicating that elevated eosinophil counts may contribute to inflammatory processes beyond the lungs [2, 5, 23]. For instance, Colon et al. demonstrated that eosinophils can drive renal fibrosis through immune activation pathways [24], which parallels our observation that COPD patients with BEC ≥ 300 cells/µL have higher odds of renal dysfunction. Likewise, Roversi et al. highlighted that systemic inflammation in COPD is associated with increased odds of cardiovascular disease [5, 25], and our results corroborate this connection by showing significant associations between elevated BEC and cardiovascular comorbidities, particularly congestive heart failure. However, our results diverge from some earlier reports. Several studies have identified significant links between peripheral eosinophilia and pulmonary comorbidities such as coexistent asthma, chronic bronchitis, or emphysema [26]. In contrast, we did not observe any such associations in our cohort after adjusting for potential confounders. This discrepancy might be attributable to heterogeneity in COPD phenotypes or to treatment-related confounding. For example, patients with higher eosinophil counts often receive intensified inhaled corticosteroid therapy, which could suppress eosinophil-driven pulmonary inflammation and thereby obscure potential links between BEC and pulmonary comorbidities.
Eosinophils are a well-established biomarker of airway inflammation, and BEC is increasingly recognized as a key parameter in guiding COPD treatment decisions and predicting therapeutic responses [27,28,29,30]. The threshold of BEC ≥ 300 cells/µL has been widely adopted in clinical practice to optimize anti-inflammatory strategies, including the selection of biologic therapies. Clinical trials targeting eosinophilic pathways have demonstrated that reducing eosinophil activity leads to improved outcomes, such as a reduction in exacerbation frequency and enhanced lung function [2, 10, 11]. Our findings build upon this framework, suggesting that BEC may also serve as a marker associated with extrapulmonary comorbidities in COPD. This highlights the potential of a BEC-guided, personalized treatment approach that not only optimizes airway inflammation management but also helps mitigate systemic complications, ultimately improving long-term prognosis.
To further evaluate potential trends in the association between BEC and comorbidities, we performed sensitivity analyses using both 3-level and 4-level BEC categories. The results aligned with our primary findings: there was a statistically significant trend showing that higher BEC levels were associated with increased odds of having at least one extrapulmonary comorbidity, while the prevalence of pulmonary comorbidities declined with rising BEC. These findings suggest a potential graded association between BEC and systemic inflammation, and support the robustness of our conclusions across different stratification strategies.
This study has several notable strengths, including the use of a large, nationally representative NHANES cohort, robust statistical modeling, and comprehensive adjustment for potential confounders. However, certain limitations should be acknowledged. First, the cross-sectional nature of NHANES precludes the establishment of causal relationships between BEC and comorbidities. In addition, NHANES does not collect information on COPD treatments, including the use of systemic or inhaled corticosteroids or biologic therapies. Since both corticosteroid and biologic exposure can significantly influence BEC levels and related clinical outcomes, the absence of treatment data represents a potential source of unmeasured confounding. Moreover, NHANES does not include data on the frequency or severity of COPD exacerbations, which are important factors known to influence cardiovascular outcomes, including congestive heart failure. Therefore, the associations observed in this study should be interpreted with caution, as they may be partly mediated or modified by differences in treatment exposure or exacerbation history. In addition, as smoking may affect eosinophil levels and comorbidities, we adjusted for smoking status to reduce residual confounding. Future longitudinal studies incorporating detailed treatment and exacerbation data are essential to validate our findings and inform clinical practice. Second, our analysis relied on a single measurement of BEC, which may not fully reflect temporal variability. This limitation could lead to phenotype misclassification in some individuals. However, previous studies suggest that BEC is relatively stable over time in many COPD patients. Oshagbemi et al. [31] reported that eosinophil stability in COPD was approximately 85% at 6 months and 75% at 1 years, with higher stability among those with low baseline levels. Stability was also greater in younger individuals and in females. These findings support the utility of single-point eosinophil measurement for population-level phenotypic stratification, while also highlighting the need for reassessment in longitudinal clinical management. Third, COPD was identified based on self-reported physician diagnosis, consistent with NHANES methodology. While this is a commonly used approach in population-based research, it does not incorporate internationally standardized spirometric criteria and may lead to misclassification. This limitation, along with potential recall or reporting bias, should be considered when interpreting the findings. Nonetheless, previous studies have supported the validity of self-reported diagnoses in large-scale epidemiological settings [32, 33]. Fourth, mortality is a critical outcome in COPD research, and its association with blood eosinophil levels may have important clinical implications. Oshagbemi et al. [34], using a large UK cohort, found that among ICS users, relative eosinophil counts ≥ 2% were associated with 12–24% lower all-cause mortality compared to < 2%. As our study was cross-sectional and focused on comorbidities, we did not evaluate mortality, nor was it a predefined endpoint. Future longitudinal studies are warranted to explore eosinophil levels in relation to survival outcomes. Finally, due to the lack of baseline matching between BEC ≥ 300 and < 300 cells/µL groups, unmeasured or residual confounding may persist despite multivariable adjustment. Patients with higher BEC may have had a greater underlying burden of comorbidities, which could bias the observed associations. This limitation should be considered when interpreting our findings.
In summary, higher BEC (≥ 300 cells/µL) in COPD was significantly associated with extrapulmonary comorbidities, particularly congestive heart failure and renal dysfunction, whereas no significant associations were observed with pulmonary comorbidities. These findings underscore the clinical relevance of eosinophilic inflammation beyond the lungs and suggest that targeted anti-eosinophilic therapies may play a role in mitigating systemic complications in COPD. To further substantiate these results, prospective studies are needed to clarify the temporal and mechanistic links between BEC and extrapulmonary comorbidities.
The data utilized in this study are derived from the NHANES 2013–2018 dataset, which is publicly accessible and downloadable from the NHANES website at http://www.cdc.gov/nchs/nhanes.htm.
- COPD:
-
Chronic obstructive pulmonary disease
- BEC:
-
Blood eosinophil count
- NHANES:
-
National health and nutrition examination survey
- NCHS:
-
National centers for health statistics
- OR:
-
Odds ratio
- CI:
-
Confidence interval
Not applicable.
This work was funded by the Chengdu Key Research and Development Support Program (grant number 2024-YF09-00021-SN).
The data analyzed in this study were derived from the National Health and Nutrition Examination Survey (NHANES). The study protocol received ethical approval from the Institutional Review Board of the National Center for Health Statistics (NCHS), with written informed consent obtained from all participants.
Not applicable.
The authors declare no competing interests.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Below is the link to the electronic supplementary material.
: Additional file 1: Table S1. Definitions and codes of variables from NHANES. Table S2. Prevalence and trend analysis of comorbidities across BEC categories (3- categories). Table S3. Prevalence and trend analysis of comorbidities across BEC categories (4- categories) Figure S1. Associations between BEC category (≥ 300 vs. < 300 cells/µL) and comorbidities in COPD participants, unadjusted model. Figure S2. Associations between BEC category (≥ 300 vs. < 300 cells/µL) and comorbidities in COPD participants, minimally adjusted model. Figure S3. Prevalence of having ≥ 1 comorbidity in COPD participants across stratified BEC levels.
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
Chen, H., Hu, X., He, C. et al. Association of blood eosinophil counts with pulmonary and extrapulmonary comorbidities in patients with chronic obstructive pulmonary disease: data from NHANES 2013–2018. BMC Pulm Med 25, 256 (2025). https://doi.org/10.1186/s12890-025-03734-6